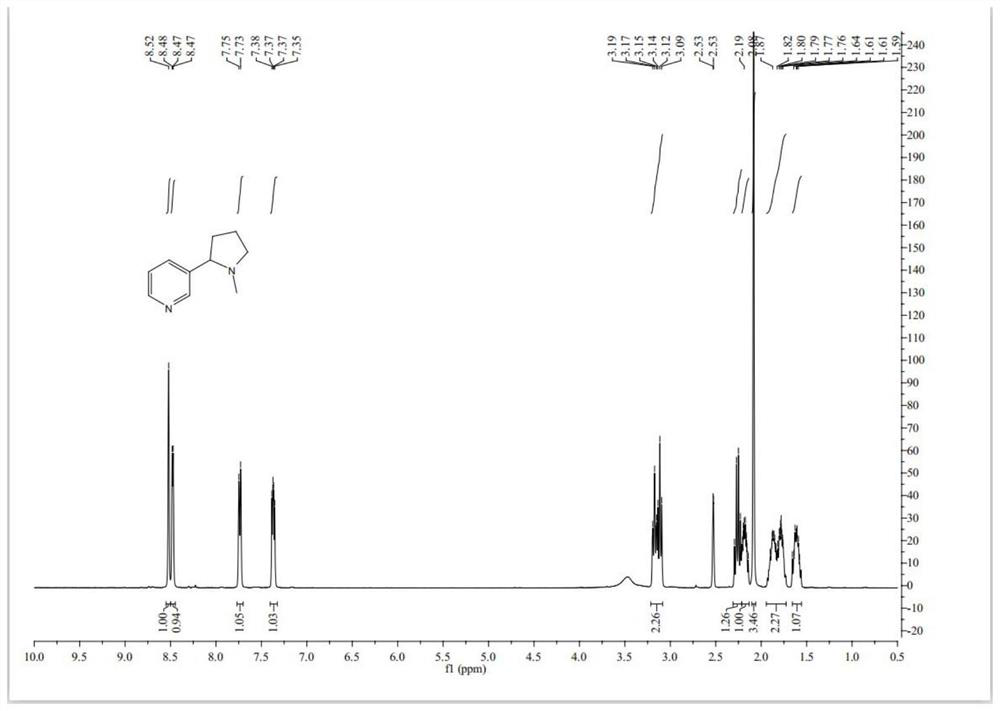
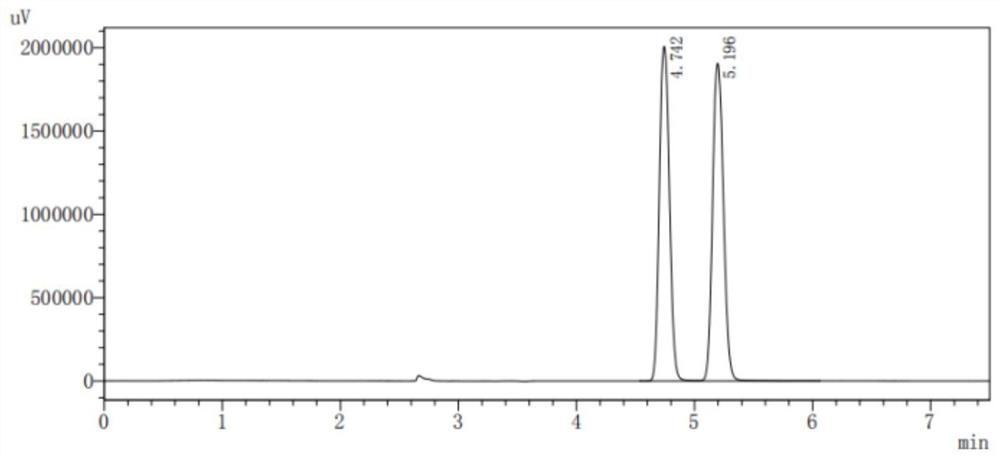
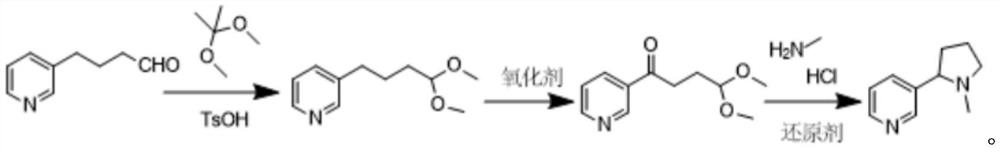
Preparation method of high-purity racemic nicotine
A technology of racemic nicotine and nicotine, which is applied in the field of preparation of high-purity racemic nicotine, can solve problems such as difficulty in large-scale production, potential safety hazards, and inability to meet large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) Dissolve 50g of 4-(pyridin-3-yl)-1-butyraldehyde, 4g of p-toluenesulfonic acid, and 70g of 2,2-dimethoxypropane in 800ml of methanol, react at 25°C, pass TLC monitors the completion of the reaction. After the 8h reaction finishes, methanol is reclaimed under reduced pressure, and the product is dissolved in 300ml of ethyl acetate. After adding 100ml of 40% aqueous sodium bicarbonate solution, after washing three times, the ethyl acetate phase is dried by adding anhydrous sodium sulfate. Ethyl acetate was recovered under pressure to obtain 64.8 g of the product 3-(4,4-dimethoxybutyl)pyridine with a purity of 94.5% and a yield of 93.6%;
[0049] (2) Take 60g of 3-(4,4-dimethoxybutyl)pyridine obtained in step (1), suspend 102.2g of selenium dioxide in 1L of dioxane, heat to reflux at 105°C, and monitor the reaction by TLC Completeness, after 12 hours of reaction, cool to room temperature, filter solid impurities, concentrate under reduced pressure and recover dioxane t...
Embodiment 2
[0057] Other operation is identical with embodiment 1, and difference is that oxidant is 66.4g calcium peroxide in step (2). Step (2) obtained 51.8 g of the product 3-(4,4-dimethoxybutyl)pyridine with a purity of 93.7%, and the yield of the oxidation step in step (2) was 79.8%.
Embodiment 3
[0059] Other operation is identical with embodiment 1, and difference is the mixture that oxygenant is 51.1g selenium dioxide and 33.2g calcium peroxide in the step (2). The final step (2) obtained 55.3 g of the product 3-(4,4-dimethoxybutyl)pyridine with a purity of 93.2%, and the yield of the oxidation step in step (2) was 84.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com