7alpha-methyl fused ring morphine derivative as well as preparation method and application thereof
A technology of fused ring and morphine, applied in the field of 7α-methyl fused ring morphine derivatives and its preparation
- Summary
- Abstract
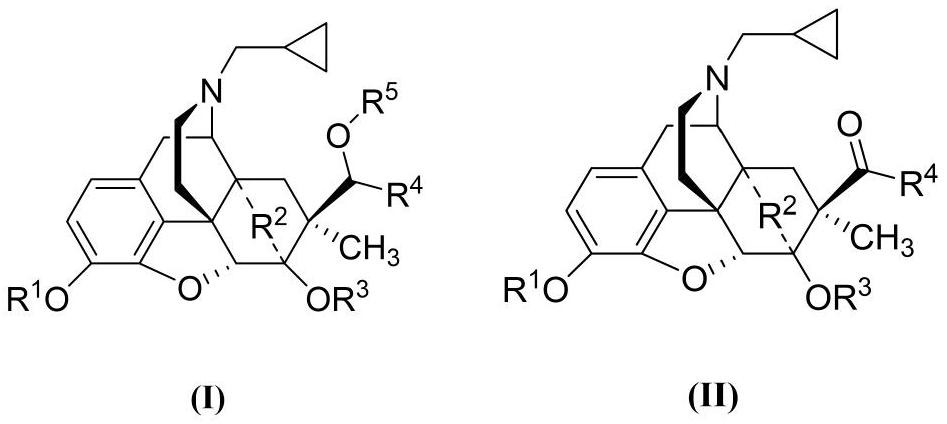
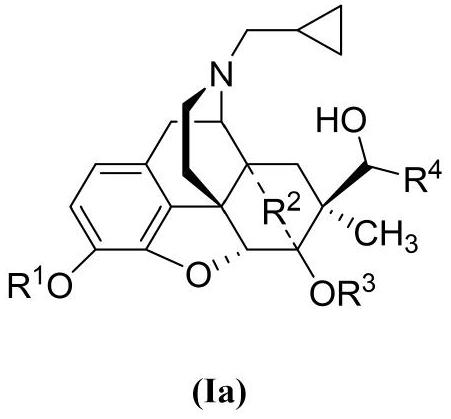
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
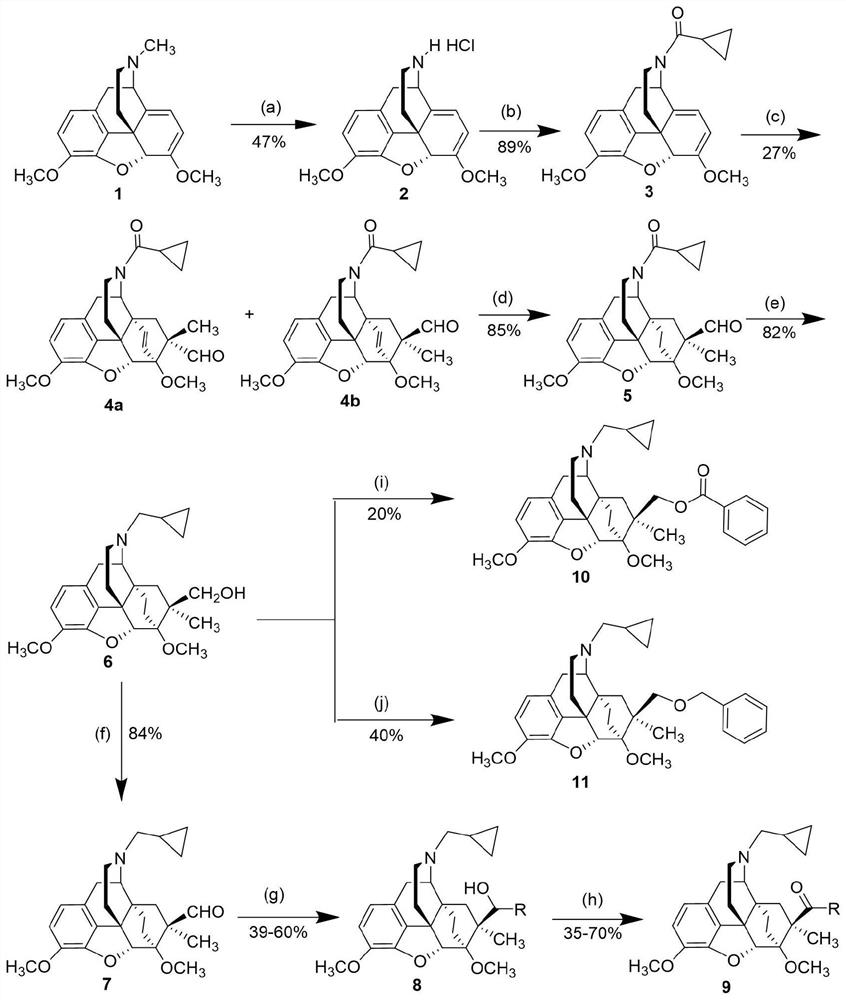
[0031] N-cyclopropylmethyl-7α-methyl-7β-hydroxymethyl-6α,14α-endo-ethylene-tetrahydronorthebaine
[0032]
[0033] Reaction route:
[0034]
[0035] Reaction conditions: (a) DIAD / acetonitrile, reflux, 4h; pyridine hydrochloride, methanol, room temperature, 2 days; (b) cyclopropanoyl chloride, triethylamine, dichloromethane, room temperature, 12h; (c) formaldehyde acrolein, lithium tetrafluoroborate, room temperature, 28h; (d) hydrogen, palladium carbon, ethanol, room temperature, 8h; (e) lithium aluminum hydride, tetrahydrofuran, room temperature, 2h.
[0036] Reaction steps:
[0037] a) Add thebaine (1g, 3.2mmol, 1eq) into a 50mL three-neck flask, then add anhydrous acetonitrile (10mL) to dissolve it, replace it with argon three times, and slowly drop it into the above solution using a dropping funnel Diisopropyl azodicarboxylate (0.68mL, 3.5mmol, 1.1eq) was added, and after the dropwise addition, the mixture was refluxed for 4h and cooled to room temperature. The solv...
Embodiment 2
[0043] N-Cyclopropylmethyl-7α-methyl-7β-aldehyde-6α,14α-endo-ethylene-tetrahydronorthebaine
[0044]
[0045] synthetic route:
[0046]
[0047] Reaction conditions: (f) oxalyl chloride, dimethyl sulfoxide, dichloromethane, triethylamine, -78°C to room temperature, 2h.
[0048] Reaction steps:
[0049] f) Add oxalyl chloride (0.06mL, 0.74mmol, 1.25eq) and anhydrous dichloromethane (3mL) into an eggplant-shaped flask, cool to -78°C, add anhydrous dimethyl sulfoxide (0.11mL, 1.53 mmol, 2.6eq) was dissolved in anhydrous dichloromethane (2mL), stirred for 5min; to the above mixture was added a solution of compound 6 (250mg, 0.59mmol, 1eq) dissolved in anhydrous dichloromethane (1mL), Stir for 20min; then add triethylamine (0.41mL, 2.94mmol, 5eq), remove the -78°C dry ice-acetone bath, and react at room temperature for 2h. The reaction solution was washed successively with saturated ammonium chloride solution (5mL x 3) and saturated sodium bicarbonate solution (5mL x 3), t...
Embodiment 3
[0051] (20R)-N-Cyclopropylmethyl-7α-methyl-7β-(1-phenyl-hydroxymethyl)-6α,14α-endo-ethylene-tetrahydronorthebaine
[0052]
[0053] synthetic route:
[0054]
[0055] Reaction conditions: (g) halides, n-butyllithium, tetrahydrofuran, -78°C, 0.25h.
[0056] Reaction steps:
[0057] g) Add bromobenzene (188mg, 1.2mmol, 10eq) and anhydrous tetrahydrofuran (2.5mL) into a Schlenk tube, cool to -78°C, replace argon three times, and add dropwise to the above reaction solution under the protection of argon Add n-butyllithium (2.4M in n-hexane, 0.5mL, 1.2mmol, 10eq), stir for 10min, then slowly inject compound 7 (50mg, 0.12mmol, 1eq) into anhydrous tetrahydrofuran solution (1mL), keep Reaction at -78°C for 0.25h. Remove the dry ice-acetone bath, quench with saturated ammonium chloride solution, add ethyl acetate (10mL x 3) and water (10mL) for extraction, combine the organic phases, wash with saturated brine (15mL), and add anhydrous sodium sulfate After drying and filtering,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com