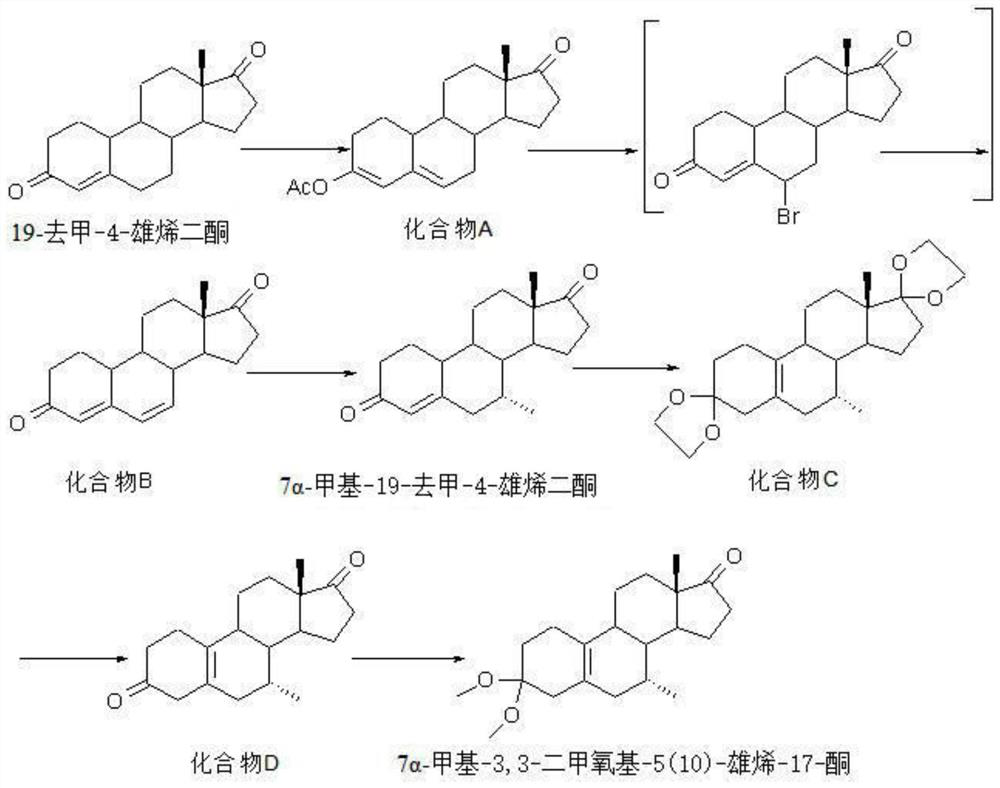
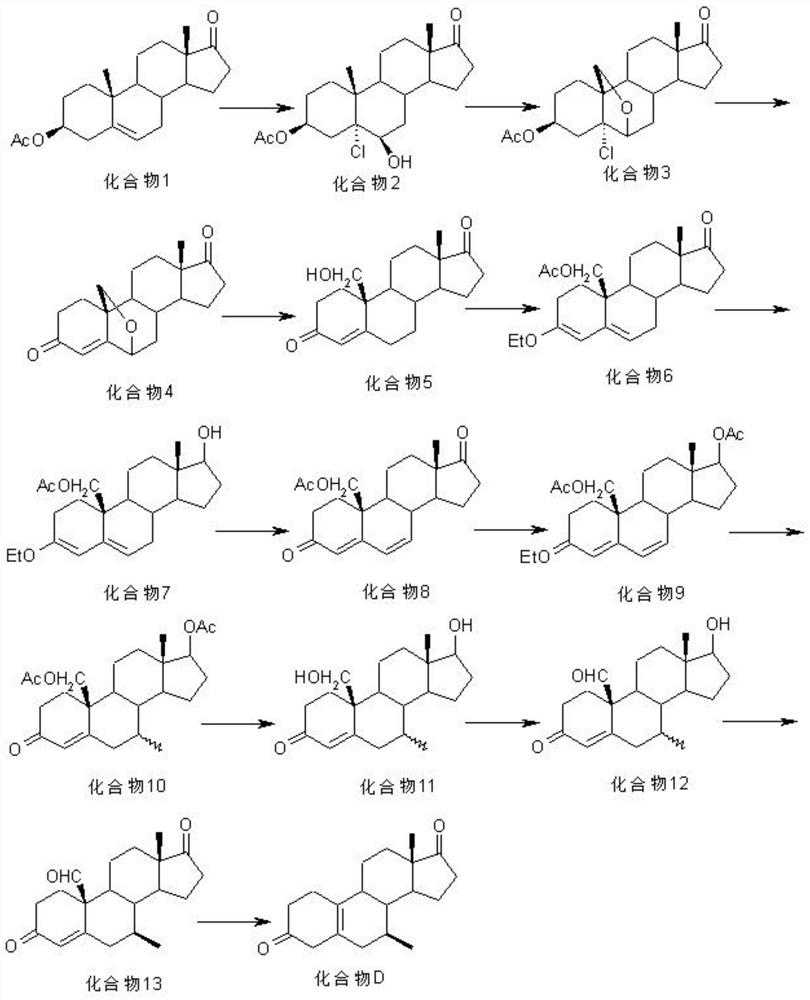
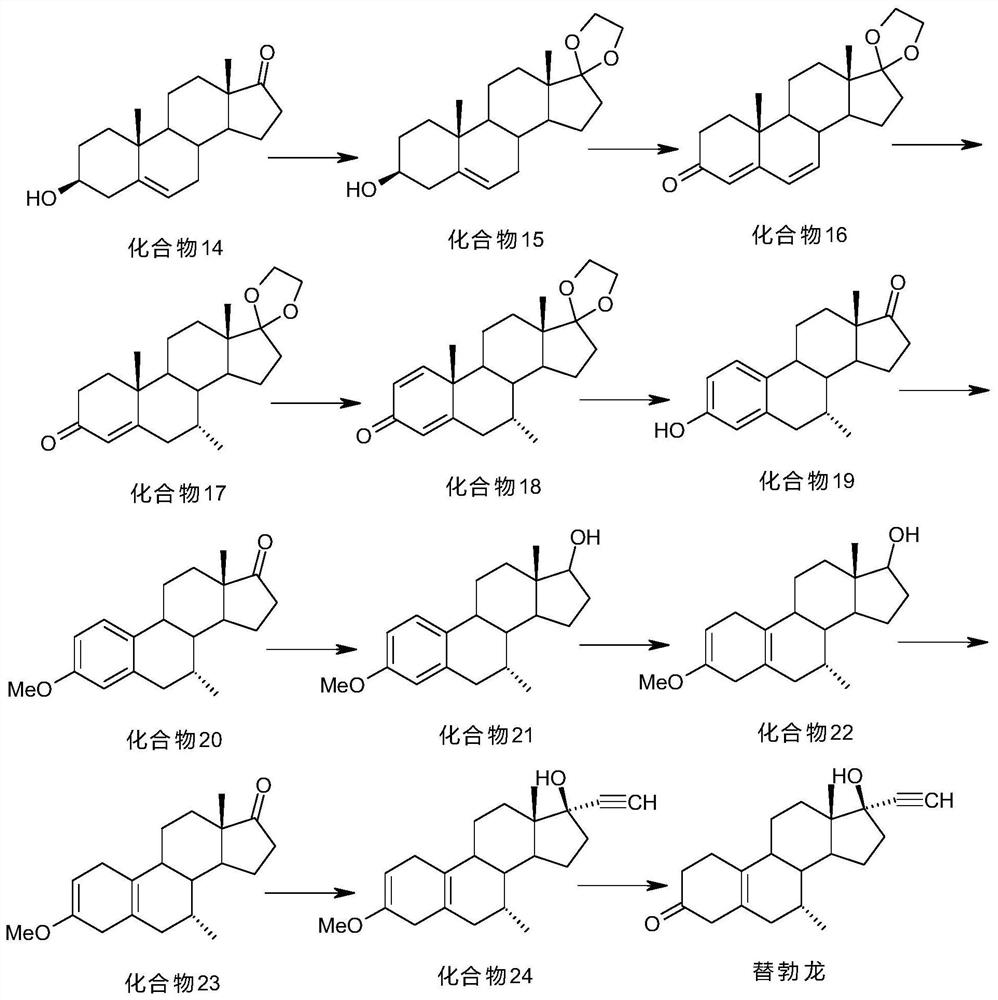
Preparation method of 7 alpha-methyl-3, 3-dimethoxy-5 (10)-androstene-17-ketone
A technology of dimethoxyl and androstenedione, which is applied in the fields of biomedicine and chemical synthesis, can solve the problems of high price, high synthesis cost, and unsuitability for industrial production, and achieve low production cost, stable and reliable quality, and high yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Preparation of Compound A:
[0085] First, 20kg of 19-nor-4-androstenedione and 80L of acetic anhydride (86.4kg) were added into the reaction kettle and mixed evenly. Then 0.4kg of p-toluenesulfonic acid was added and the reaction was stirred at room temperature for 5h. After the reaction was complete as detected by TLC, the reaction solution was poured into ice water for crystallization, centrifugally filtered, the filter cake was washed 3 times with water, and dried to obtain 23kg of white crystalline compound A with a yield of 115%, Mp: 149-151°C.
Embodiment 2
[0087] Preparation of Compound A:
[0088] First, 20kg of 19-nor-4-androstenedione and 80L of acetic anhydride (86.4kg) were added into the reaction kettle and mixed evenly. Then add 0.5kg pyridine hydrochloride and stir the reaction at room temperature for 5h. After the reaction was complete as detected by TLC, the reaction solution was poured into ice water for crystallization, centrifugally filtered, the filter cake was washed 3 times with water, and dried to obtain 21kg of white crystalline compound A with a yield of 105%, Mp: 149-151°C.
Embodiment 3
[0090] Preparation of Compound B:
[0091] 10kg of compound A was dissolved in 40LN,N-dimethylformamide (37.92kg), cooled to 0°C, then 6kg of N-bromosuccinimide was added and the reaction was stirred at room temperature for 1h, and the reaction was detected by TCL until the end of the reaction. Then add 8 kg of calcium carbonate to continue the reaction at a temperature of 80° C., and TCL detects that the reaction ends. After filtration, the filtrate was poured into a sufficient amount of ice water for crystallization, and then filtered to obtain 6.7kg of light yellow crystalline compound B with a yield of 67%, mp: 178-180°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com