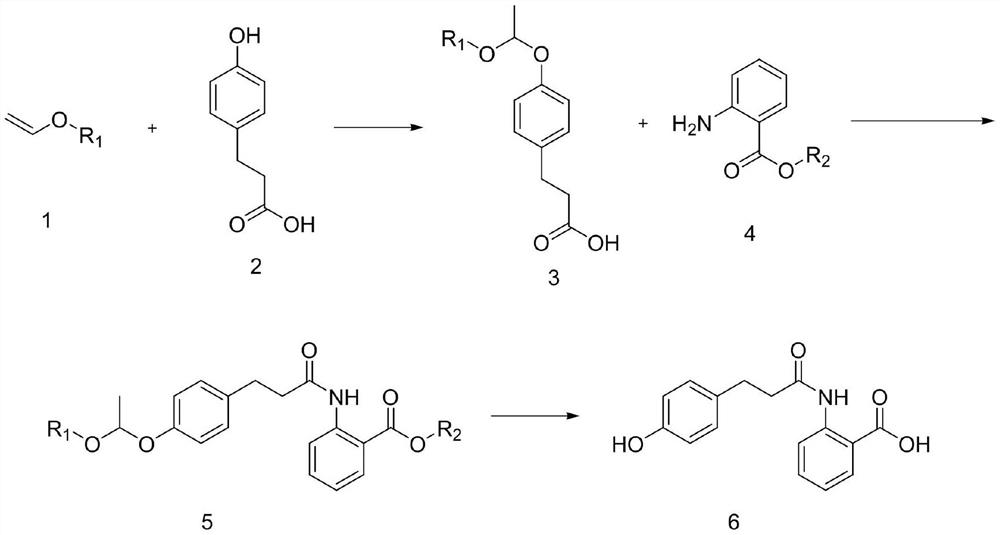
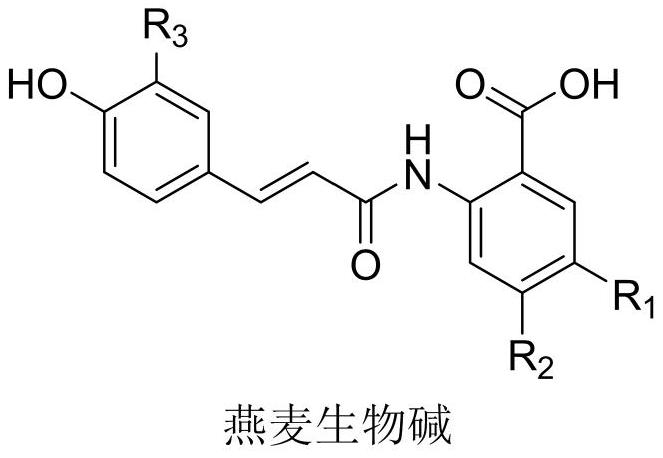
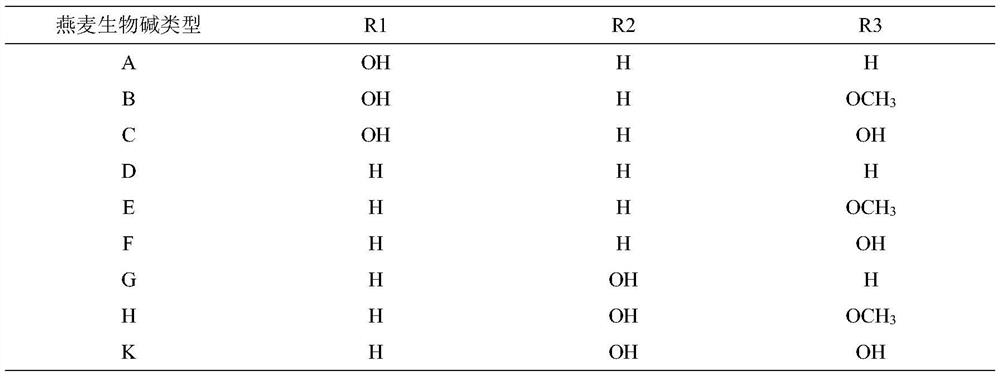
Preparation method of dihydrooat alkaloid
A technology of oat alkaloids and dihydrogen, which is applied in the field of preparation of dihydrooat alkaloids, can solve problems such as corrosion and expensive acetoxyphenylpropionate, and achieve the effects of high reaction purity, low reaction temperature, and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037] Into the reaction flask, put 33.24g (0.2mol) of p-hydroxyphenylpropionic acid, 150mL of dichloromethane and 5.0g (0.02mol) of pyridinium p-toluenesulfonate, and slowly add 15.87g (0.22mol) of p-toluenesulfonate at a controlled temperature of 15-20°C After the addition of vinyl ether / dichloromethane mixed solution, control the temperature at 20-25 ° C for 5 hours, TLC detects that there is no raw material remaining, wash with saturated saline solution, and concentrate the organic phase to no liquid to obtain 3-(4- (1-Ethoxyethoxy)phenyl)propanoic acid 46.08g, yield 96.7%, HPLC 95.3%. 1 HNMR (400MHz, CDCl 3 ):11.93(s,1H),7.15-7.11(m,2H),6.87-6.84(m,2H),5.60-5.65(m,1H),3.86-3.82(m,2H),2.73-2.69(m ,2H),2.51-2.48(m,2H),1.61-1.58(m,3H),1.20-1.17(m,3H).
Embodiment 2
[0039]
[0040] Into the reaction flask, put 33.24g (0.2mol) of p-hydroxyphenylpropionic acid, 150mL of dichloromethane and 5.0g (0.02mol) of pyridinium p-toluenesulfonate, and slowly add 18.95g (0.22mol) of p-toluenesulfonate at a temperature of 15-20°C Vinyl isopropyl ether / dichloromethane mixed solution, after the addition is complete, control the temperature at 20-25 ° C for 5 hours, and TLC detects that there is no raw material remaining, wash with saturated saline solution, and concentrate the organic phase to no liquid to obtain 3-( 4-(1-isopropoxyethoxy)phenyl)propanoic acid 49.0g, yield 97.1%, HPLC 94.8%.
Embodiment 3
[0042]
[0043] Into the reaction bottle, put 33.24g (0.2mol) of p-hydroxyphenylpropionic acid, 150mL of dichloromethane and 5.0g (0.02mol) of pyridinium p-toluenesulfonate, and slowly add 18.95g (0.22mol) ) Vinyl n-propyl ether / dichloromethane mixed solution, after the addition, control the temperature at 20-25°C for 5 hours of reaction, TLC detects that there is no raw material remaining, washing with saturated saline solution, and concentrating the organic phase to no liquid to obtain 3- (4-(1-n-propoxyethoxy)phenyl)propanoic acid 48.5g, yield 96.1%, HPLC 95.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com