Synthesis and purification method of high-purity N-isobutoxy methacrylamide (IBMA)
A technology of isobutoxymethacrylamide and purification method is applied in the separation/purification of carboxylic acid amide, chemical instruments and methods, preparation of carboxylic acid amide, etc. and other problems, to achieve the effect of reducing self-aggregation behavior, convenient operation and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
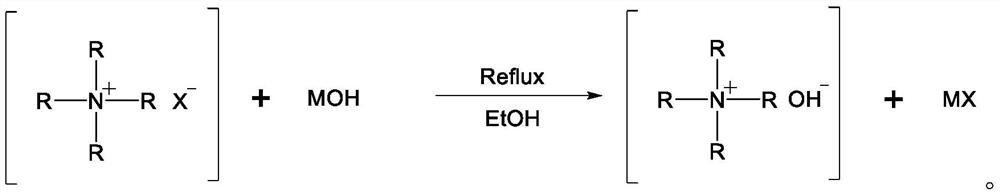
Method used
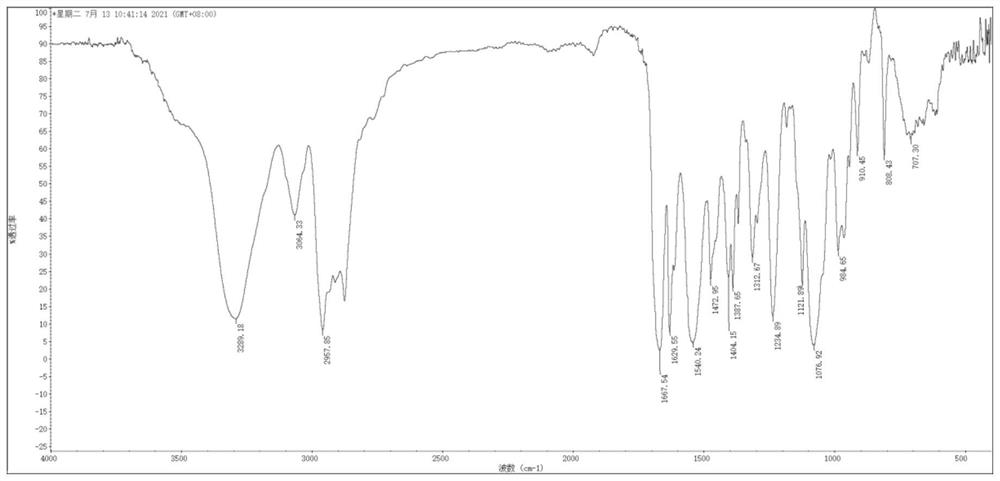
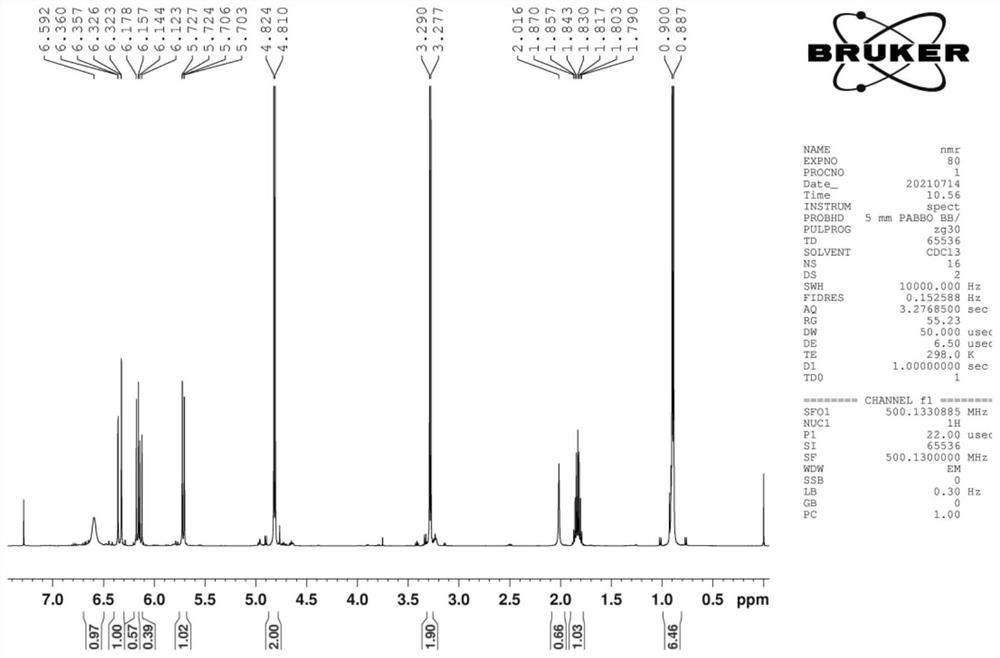
Image
Examples
Embodiment 1
[0029] Embodiment 1: This embodiment provides a synthesis and purification method of high-purity grade N-isobutoxymethacrylamide IBMA, comprising the following steps:
[0030] S1: Add 350ml of absolute ethanol to a 500ml round bottom flask, add 11g (0.1mol) of tetramethylammonium chloride, 5.2g (0.13mol) of sodium hydroxide, heat and reflux for 4 hours, cool to room temperature, and centrifuge to separate the precipitate , take 200ml of supernatant. Add about 200g of calcined and activated aluminum oxide (α-Al 2 o 3 ), ensure that the aluminum oxide is completely immersed in the solution, and stand at room temperature for 24 hours. The mesoporous alumina was filtered out, and nitrogen was purged at room temperature for 24 hours to dryness to obtain a supported quaternary ammonium base catalyst, which was stored in a vacuum desiccator for future use. The obtained catalyst was recorded as Cat Me 4 NOH / AO.
[0031] S2: In a 500ml round bottom flask, add 120g Cat Me 4 NOH / AO,...
Embodiment 2
[0035] S1: Add 350ml of absolute ethanol to a 500ml round bottom flask, add 15g (0.1mol) of tetrabutylammonium bromide, 11.2g (0.2mol) of potassium hydroxide, heat and reflux for 4 hours, cool to room temperature, and centrifuge to separate the precipitate , take 200ml of supernatant. Add about 200 g of calcined and activated aluminum oxide (α-Al2O3) to the supernatant to ensure that the aluminum oxide is completely immersed in the solution, and stand at room temperature for 24 hours. Mesoporous alumina was filtered out, and room temperature was purged with nitrogen for 24 hours to dryness to obtain a supported quaternary ammonium base catalyst, which was stored in a vacuum desiccator for future use. The obtained catalyst was designated as Cat Bu4NOH / AO.
[0036] S2: In a 500ml round bottom flask, add 150g Cat Bu4NOH / AO, add 170g deionized water, add 4.4g (0.04mol) of hydroquinone, 574g (8mol) of acrylamide and 600g (8.1mol) of isobutanol to the system mol), the temperature o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com