Tetrahydroxanthone dimer compound as well as preparation method and application thereof
A xanthone dimer and compound technology, which is applied in the field of tetrahydroxanthone dimer compounds, can solve problems such as no anti-Helicobacter pylori activity, and is convenient for pharmacological and clinical research, extraction and separation Simple method, good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
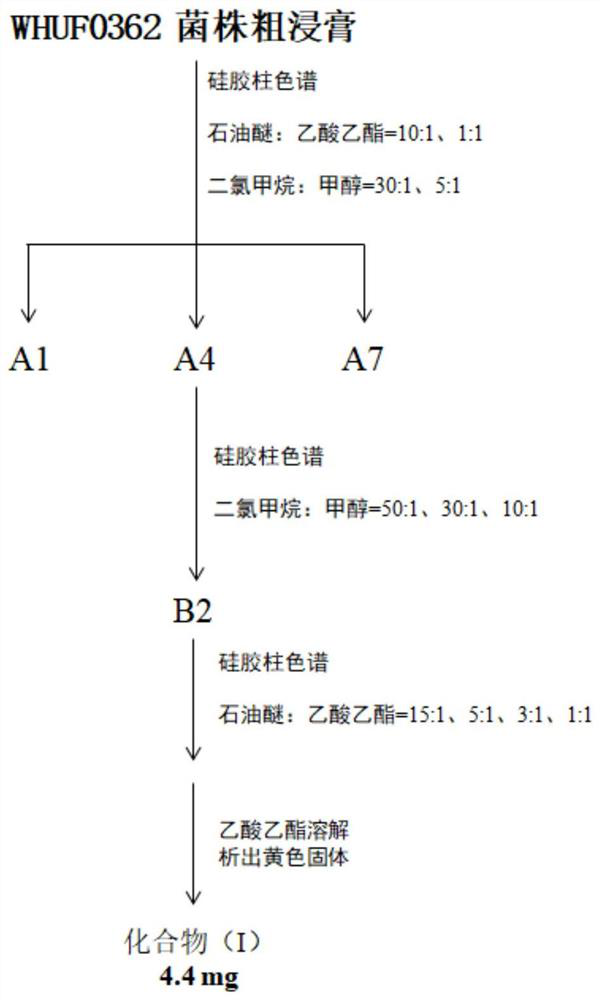
[0030] Example 1: Screening and identification of bacterial strain WHUF0362
[0031] 1. Strain screening
[0032] (1) The mangrove candela rhizosphere soil samples collected from Yalong Bay, Sanya City, Hainan, China in December 2018 were diluted step by step with sterile water to 10-1 , 10 -2 , 10 -3 For different gradients, each dilution is vortexed for 3-5 minutes before the next dilution; the samples of different dilution gradients are evenly coated on the MEA medium with a coating rod, and placed in a 30°C constant temperature incubator for upside-down culture . Three parallel experiments were set up for each sample.
[0033] (2) In the following 1 to 7 days, observe the culture of step (1) and pick different types of single colonies and repeat step (1) to streak and separate until it is determined to be a pure single colony.
[0034] (3) Inoculate the different single colonies of step (2) on PDA solid medium respectively, and after culturing at 28°C for 24 hours, cut...
Embodiment 2
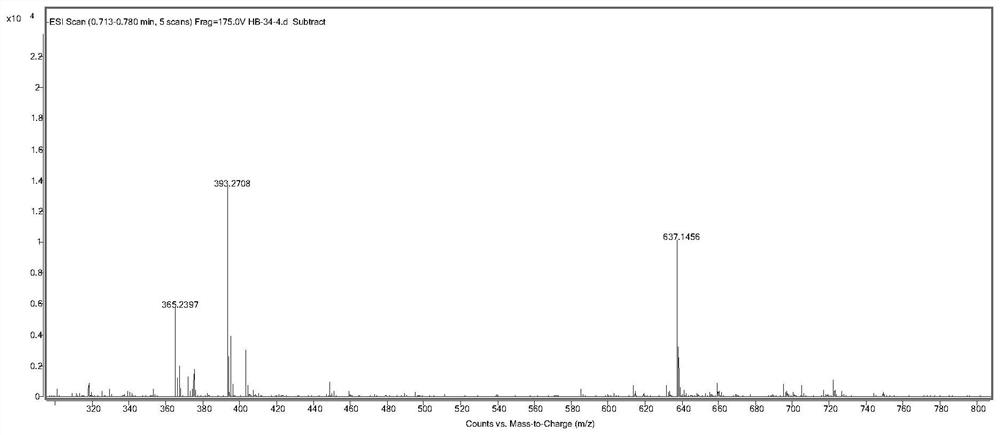
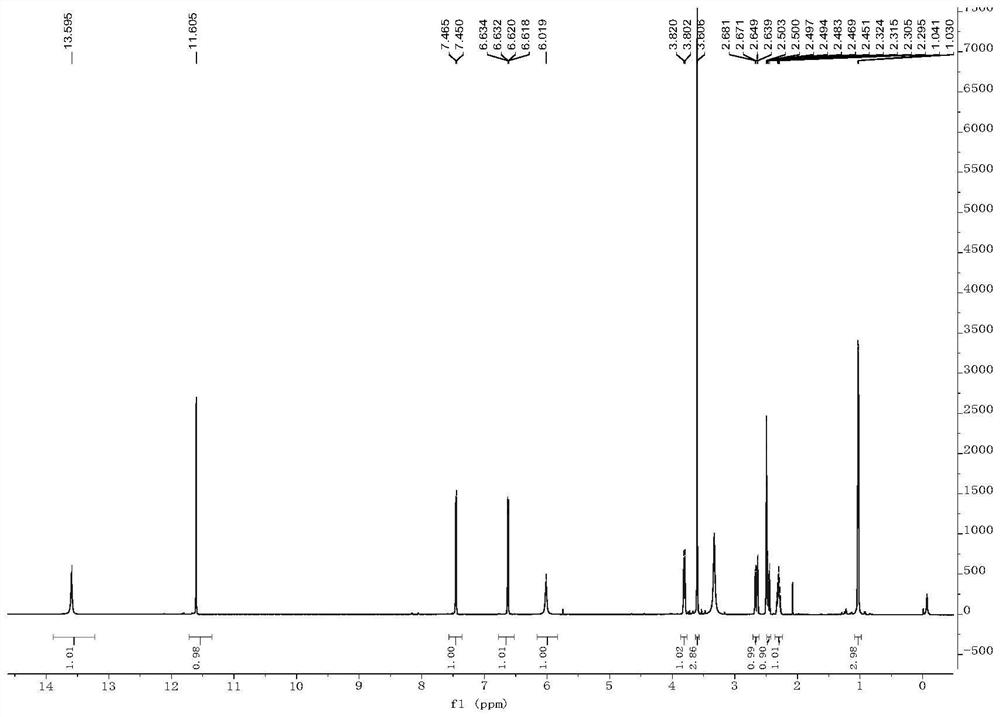
[0050] Embodiment 2, the preparation of formula (I) compound
[0051] 1. Fermentation culture
[0052] Pick the basket-shaped fungus WHUF0362 strain stored in the cryopreservation tube, inoculate it into a plate medium, and cultivate it in an incubator at 28°C for 3 days until the colony grows to maturity. Inoculate the ISP with spores 4 In the liquid medium, shake culture at 28° C. and 200 rpm for 4 days, and serve as seed solution. The seed solution was respectively inserted into sterilized 1 L Erlenmeyer flasks containing 200 g of rice culture medium with an inoculation amount of 5% volume concentration, a total of 60 flasks. The fermented product was obtained by statically culturing at room temperature for 15 days.
[0053] The final concentration of the plate medium is composed of: potato 200g / L, glucose 20g / L, agar 18g / L, solvent is water, and natural pH.
[0054] ISP 4 The final concentration of the liquid medium consists of: soluble starch 15g / L, glucose 5g / L, pep...
Embodiment 3
[0074] Embodiment 3: Formula (I) compound antibacterial activity in vitro
[0075] (1) Preparation of the test bacteria solution: select the monoclonal colony of Helicobacter pylori 26695 (H. pylori26695) grown on the solid medium, inoculate it on the Columbia plate medium, culture it at 37°C for 2 days and grow to the stationary phase, then Inoculate into fresh BHI medium containing 10% calf serum in a volume ratio of 1:100, and culture at 37°C for 1d to mid-late logarithmic (OD 600nm =0.8-1.0), the obtained concentration is 10 6 CFU / mL of bacterial liquid.
[0076] Preparation of Helicobacter pylori G27 (H.pylori G27), Helicobacter pylori 159 (H.pylori 159) and Helicobacter pylori 129 (H.pylori 129) bacteria liquid: Helicobacter pylori 26695 were replaced with Helicobacter pylori G27 (H.pylori G27, purchased from American Type Culture Collection ACTT), Helicobacter pylori 159 (H.pylori 159, purchased from American Type Culture Collection ACTT) and Helicobacter pylori 129 (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com