Arginase inhibitor as well as pharmaceutical composition and application thereof
An arginase and inhibitor technology, applied in the field of its pharmaceutical composition and arginase inhibitor, can solve the problems of poor activity, high cost of drug preparation and medication, insufficient treatment convenience, etc., and achieve good safety It has the advantages of high stability, easy promotion and production, and low preparation cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The inhibitory activity assay of embodiment 1 arginase inhibitor to arginase
[0037] (1) Draw a urea standard curve: Accurately weigh 10 mg of urea, and prepare 0.1, 0.2, 0.4, 0.8, and 1.2 mg / mL serial concentration solutions with 50 mM Tris HCl; take 50 μL of urea solutions with different concentrations, and add 43 μL of 50 mM Tris HCl respectively , 75μL 10mmol / L MnCl 2 (50mM Tris·HCl preparation), 50μL arginase substrate (0.5mol / L L-arginine, pH9.7), 200μL acid solution (H 2 SO 4 :H 3 PO 4 :H 2O, 1:3:7), 25 μL of 9% 2-isonitrosopropiophenone (prepared in absolute ethanol), heated at 100°C for 45min, and then placed the sample in the dark at room temperature for 10min, and used an automatic microplate reader to The analytical system measures absorbance at 550 nm. The standard curve was obtained by taking the concentration of urea solution as the abscissa and the absorbance value as the ordinate.
[0038] (2) Take 2 mg of arginase, prepare it to 2 mg / mL with 50...
Embodiment 2
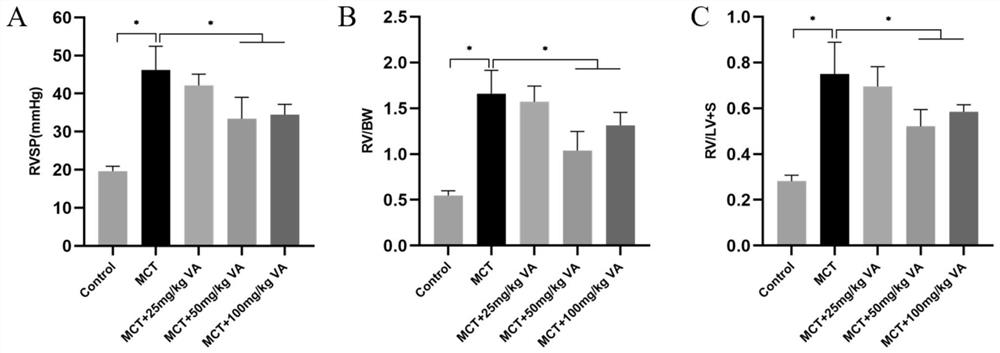
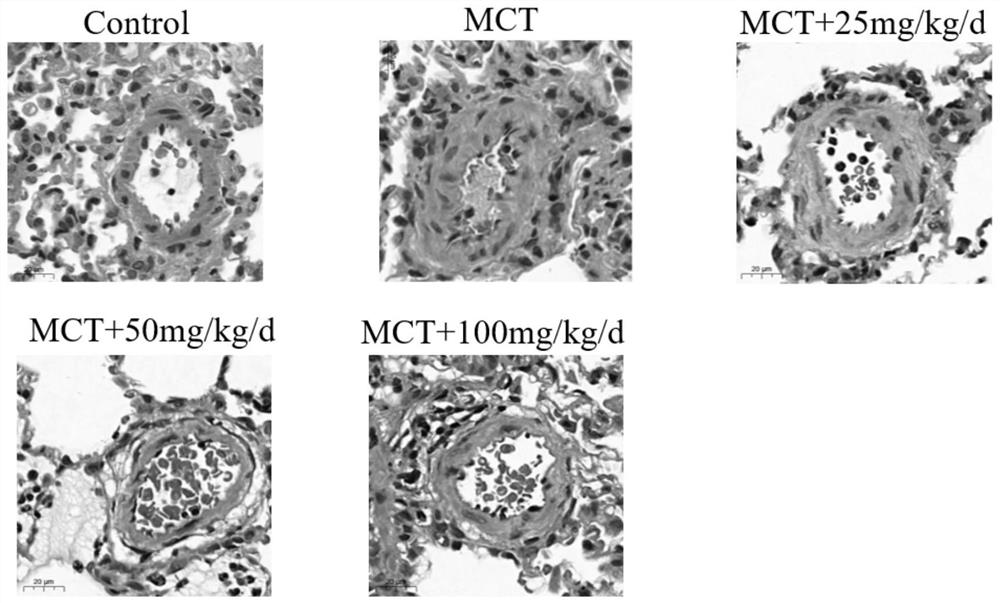
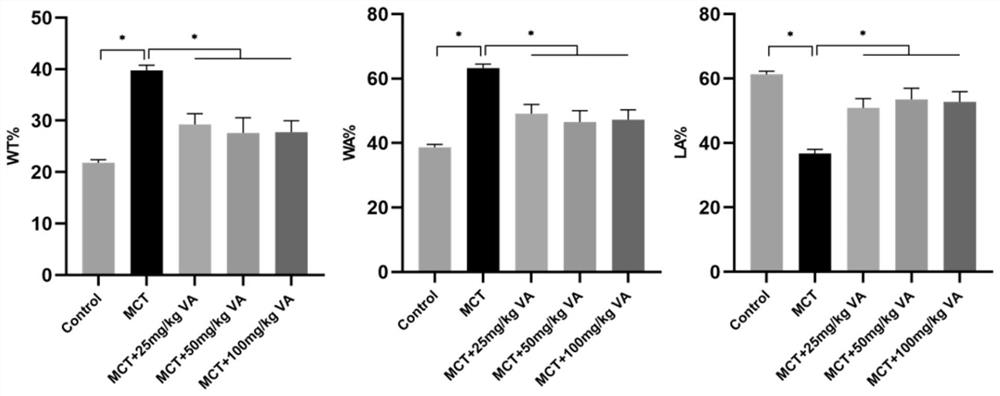
[0045] Example 2 Intervention of Arginase Inhibitor Vanillic Acid on Rat Pulmonary Hypertension
[0046] (1) Establish a rat model of pulmonary arterial hypertension by one-time subcutaneous injection of 40 mg / kg monocrotaline, grouped into: control group, MCT model group and drug treatment group rats, from the first day of rat injection of monocrotaline , orally administered three different doses of vanillic acid (25mg / kg / d, 50mg / kg / d and 100mg / kg / d), and 10 experimental rats in each group were fed for 28 days.
[0047] (2) At 28 days, analyze and evaluate its efficacy from body weight, right ventricular pressure, hematological indicators, right heart hypertrophy index, etc. The right ventricular pressure (RVSP) of the rats in each group was measured with a Power Lab physiological recorder and a biological acquisition system using the venous catheter method. After dissection, the rat heart was removed, and the right ventricle was isolated along the interventricular septum, w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com