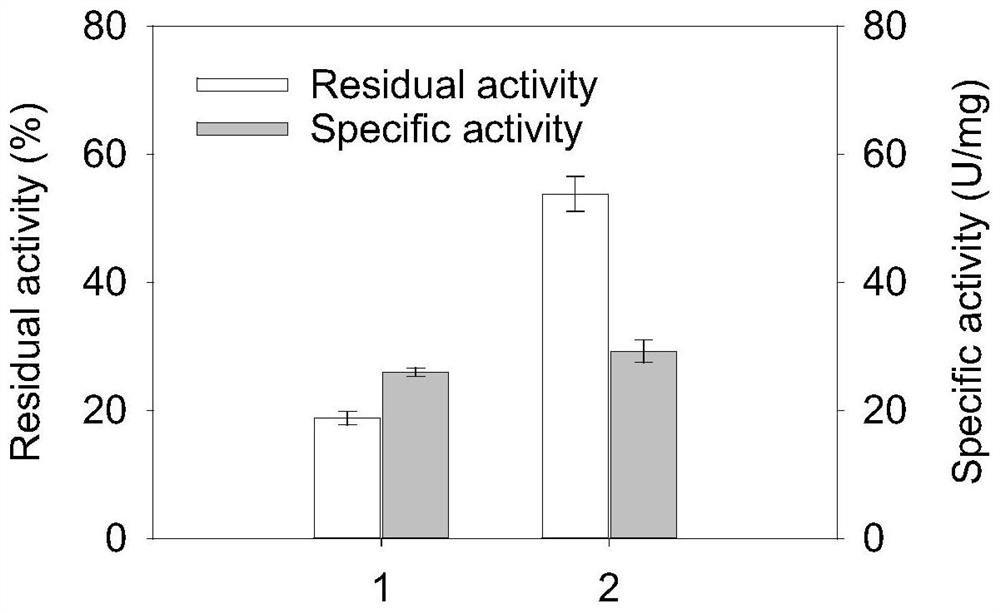
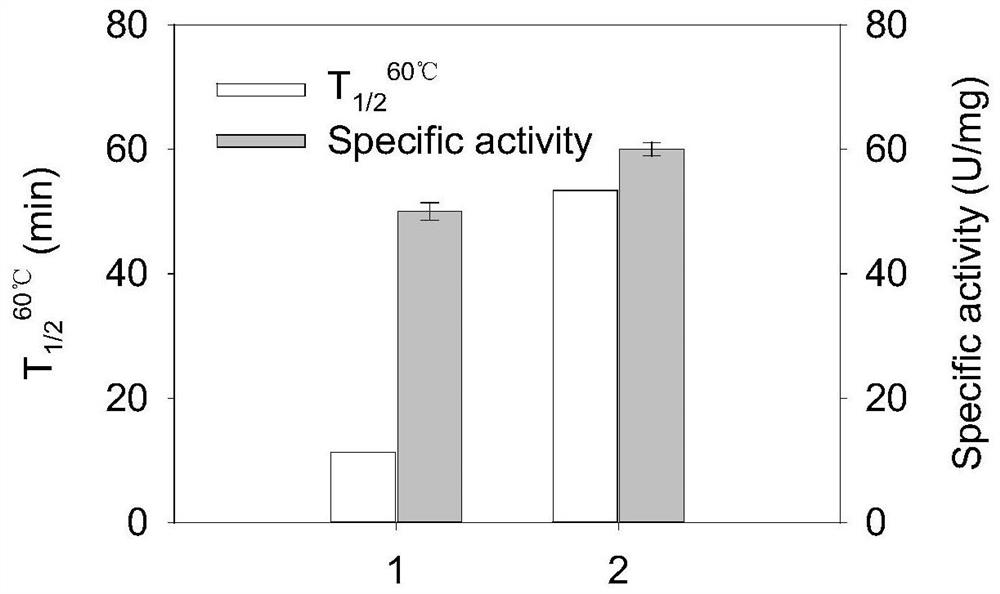
Glutamine transaminase variant with improved catalytic activity and thermal stability
A glutamine and transaminase technology, applied in the field of transglutaminase variants, can solve the problems of TGase poor stability, high cost, limited application space, etc., and achieve the effect of improving thermal stability and specific enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0076] Preparation of variants
[0077] Transglutaminase variants of the invention can be prepared using any mutagenesis procedure known in the art (eg, site-directed mutagenesis, synthetic gene construction, semi-synthetic gene construction, random mutagenesis, shuffling, etc.).
[0078] Site-directed mutagenesis is a technique for introducing one or more (eg, several) mutations at one or more defined sites in the polynucleotide encoding said parental transglutaminase.
[0079] Site-directed mutagenesis can be accomplished in vitro by PCR involving the use of oligonucleotide primers containing the desired mutation. In vitro site-directed mutagenesis can also be performed by cassette mutagenesis, which involves cleavage by restriction enzymes at a site in the plasmid containing the polynucleotide encoding the parental transglutaminase and subsequent insertion of the oligo containing the mutation into Nucleotides are linked in polynucleotides. Typically, the restriction enzym...
Embodiment 1
[0130] The preparation and thermal stability of some transglutaminase variants will be described below in conjunction with one of the specific embodiments.
[0131] The involved Escherichia coli JM109 and Escherichia coli E.coli BL21 (DE3) were purchased from takara-Baori Medical Biotechnology (Beijing) Co., Ltd., and the pET-22b (+) plasmid was purchased from Novagen (the above-mentioned strain E.coli BL21 (DE3) can be purchased and does not need to be preserved for patent procedures), neutral protease is purchased from Beijing Soleibao Technology Co., Ltd. (product number Z8032), Blunting Kination Ligation (BKL) Kit and HS DNA Polymerase was purchased from Biotech Biotechnology (Beijing) Co., Ltd., and Bradford Protein Concentration Assay Kit (detergent-compatible type) was purchased from Shanghai Biyuntian Biotechnology Co., Ltd.
[0132] The media involved are as follows:
[0133] LB liquid medium: yeast powder 5.0g / L, tryptone 10.0g / L, NaCl 10.0g / L, ampicillin 100μg / L. ...
Embodiment 2
[0152] Example 2: Application of transglutaminase variants in meat processing
[0153] Utilize the transglutaminase variant 2 mature enzyme prepared in embodiment 1 to carry out the processing of dried rabbit meat, specifically:
[0154] S1, mince the rabbit meat and dice the chicken to obtain mixed meat;
[0155] S2. After uniformly mixing salt, compound phosphate and water, adding the mixed meat obtained in S1, mixing evenly, sealing with a plastic wrap, and marinating for 10 hours to obtain cured meat;
[0156] S3. Homogenizing the marinated meat to obtain mixed minced meat;
[0157] S4. Add transglutaminase, ovalbumin, ginger powder, etc. to the mixed minced meat obtained in S3, and stir evenly at a temperature of 2° C. to obtain mixed minced meat;
[0158] S5. After sealing the mixed minced meat obtained in S4 with a plastic wrap, place it in a water bath at a temperature of 60° C. for 0.2 h, extrude, dry, and cool naturally to obtain a semi-finished product;
[0159] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com