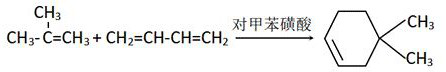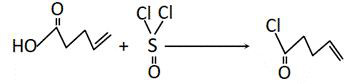Synthesis method of dimethyl cyclohexenyl pentenone
A technology of dimethylcyclohexenylpentenone and dimethylcyclohexene, which is applied in the field of chemical synthesis, can solve the problems of many operation steps, many side reactions, and environmental pollution, and achieve the optimization of raw material ratio and high yield Improvement, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
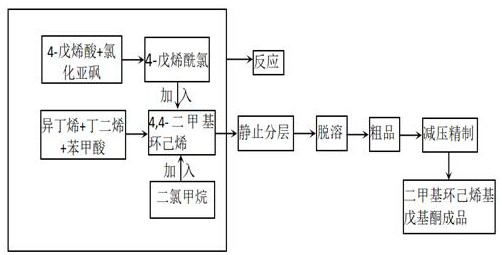
[0031] 1) Preparation of 4.4-dimethylcyclohexene;
[0032] Add 100ml of n-hexane, 274.5g of butadiene and 424.5g of isobutylene into a 1000ml autoclave, then add 1g of benzenesulfonic acid, immediately close the mouth of the autoclave to prevent gasification, stir, and maintain the temperature at -35°C and the pressure at 2.0Mpa. to 2.0MPa, pressurized with nitrogen, reacted at constant temperature for 1.5h, cooled, neutralized with saturated sodium carbonate solution, adjusted to pH 7, separated the organic layer, and precipitated to obtain 4.4-dimethylcyclohexene, mass It was 427.6 g, and the yield was 76.3%.
[0033] 2) Preparation of 4-pentenoyl chloride;
[0034] 2. Add 250ml of 4-pentenoic acid to a 1000ml three-neck round-bottomed flask, add 400g of thionyl chloride dropwise while stirring and cooling down, heat to reflux, and gradually increase the temperature as the reflux time prolongs, then raise the temperature to 100°C and react for 1 hour . Change reflux to di...
Embodiment 2
[0038] 1) Preparation of 4.4-dimethylcyclohexene;
[0039] Add 200ml of n-hexane, 550g of butadiene and 850g of isobutene into a 2000ml autoclave, then add 4g of benzenesulfonic acid, immediately close the mouth of the autoclave to prevent gasification, stir, and maintain the temperature at -30°C and the pressure at 2.0Mpa, if the pressure is less than 2.0 MPa, pressurized with nitrogen, reacted at constant temperature for 1.5h, cooled, neutralized with saturated sodium carbonate solution, adjusted pH to 7, separated the organic layer, and precipitated to obtain 4.4-dimethylcyclohexene with a mass of 800g , the yield was 71.2%.
[0040] 2) Preparation of 4-pentenoyl chloride;
[0041] Add 500ml of 4-pentenoic acid to a 2000ml three-neck round bottom flask, add 800g of thionyl chloride dropwise while stirring and cooling down, heat to reflux, and gradually increase the temperature as the reflux time prolongs, then raise the temperature to 100°C and react for 2 hours. Change r...
Embodiment 3
[0045] 1) Preparation of 4.4-dimethylcyclohexene;
[0046] Add 500ml of n-hexane, 1370g of butadiene and 2122g of isobutene into a 5000ml autoclave, then add 10g of benzenesulfonic acid, immediately close the mouth of the autoclave to prevent gasification, stir, and maintain the temperature at -40°C and the pressure at 2.0Mpa. If the pressure is less than 2.0MPa, pressurize with nitrogen, react at constant temperature for 1 hour, cool down, neutralize with saturated sodium carbonate solution, adjust the pH to 7, separate the organic layer, and desolventize to obtain 4.4-dimethylcyclohexene , the mass is 2080g, and the yield is 74.3%.
[0047] 2) Preparation of 4-pentenoyl chloride;
[0048] Add 1250ml of 4-pentenoic acid to a 5000ml three-necked round-bottomed flask, add 2000g of thionyl chloride dropwise while stirring and cooling down, heat to reflux, and gradually increase the temperature as the reflux time prolongs, then raise the temperature to 100°C and react for 1 hour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com