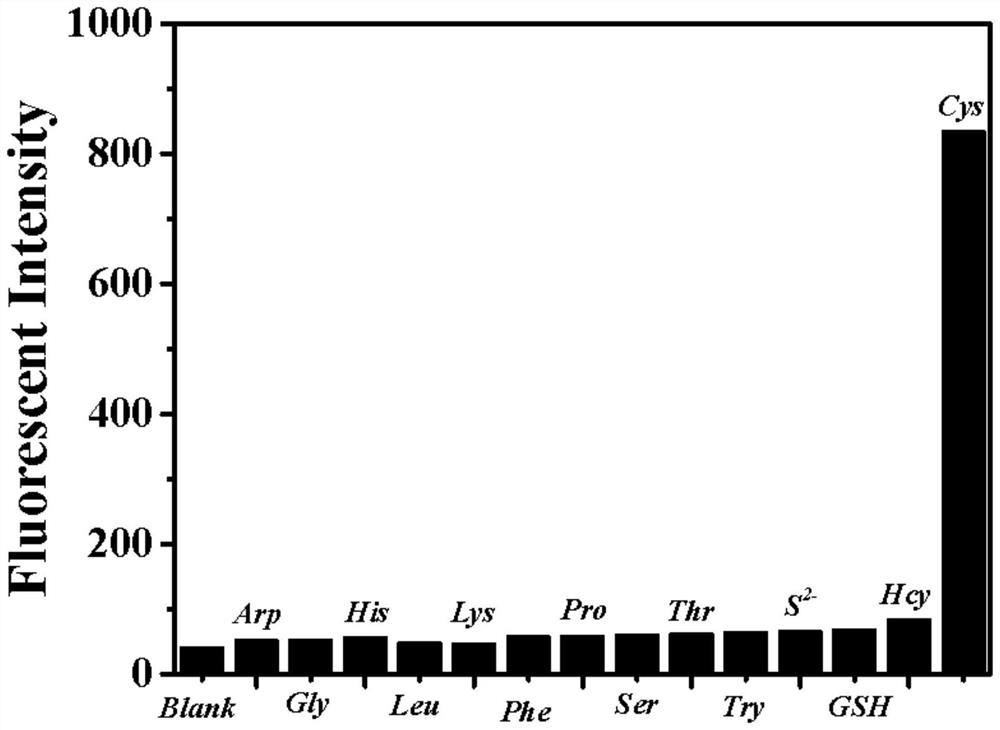
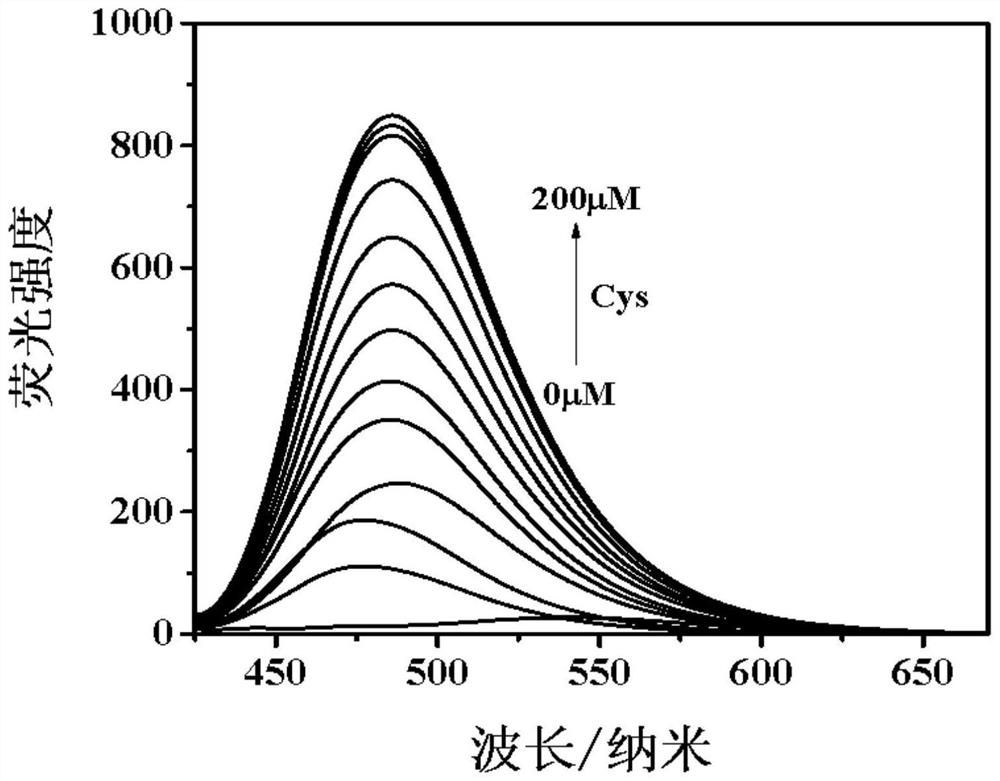
Fluorescent probe based on benzothiazole derivative cysteine and preparation method thereof
A fluorescent probe, cysteine technology, applied in the field of cysteine detection, can solve the problem of long response time, and achieve the effect of good imaging effect, low cytotoxicity and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A method for preparing a fluorescent probe based on benzothiazole derivative cysteine, the steps are as follows:
[0031] (1) Add 0.5 g of cyanobiphenol aldehyde and 0.32 g of o-aminothiophenol to absolute ethanol, then dropwise add 1.12 ml of hydrogen peroxide and 1.74 ml of concentrated hydrochloric acid, stir at room temperature for 30 min, and after the reaction is complete, suction filter to obtain a light yellow Solid II, 80% yield.
[0032]
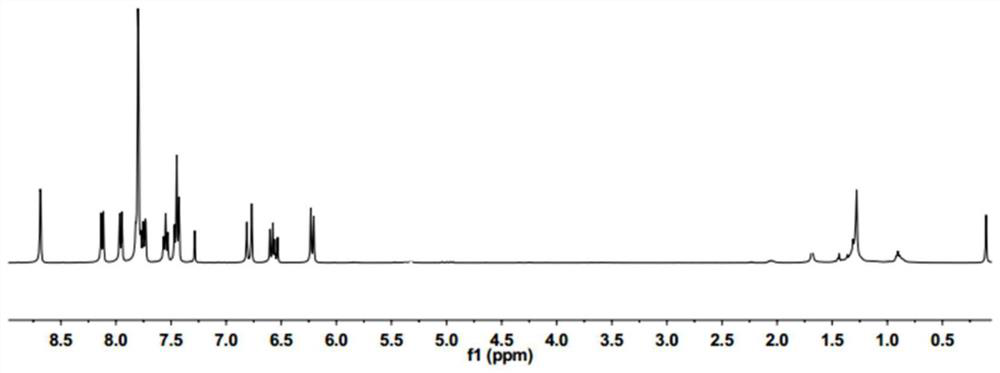
[0033] (2) Under a nitrogen atmosphere, in an ice bath, add 0.19 g of compound II and 0.16 ml of acryloyl chloride to dry dichloromethane, then dropwise add 0.2 ml of triethylamine, stir at room temperature overnight, after the reaction is complete, concentrate in vacuo to obtain crude Product, compound I was obtained by column chromatography with a yield of 55%. figure 1 It is the NMR image (400MHz, CDCl3) of the obtained probe.
[0034]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com