Efinbuprofen sustained-release tablet and preparation method thereof
A technology of efluprofen and sustained-release tablets, applied in the field of efluprofen sustained-release tablets and preparation thereof, can solve the problems of low patient compliance, bitter taste of efluprofen, and inability to achieve taste masking and the like , to achieve the effect of reducing the number of doses, not easy to burst, and reducing irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
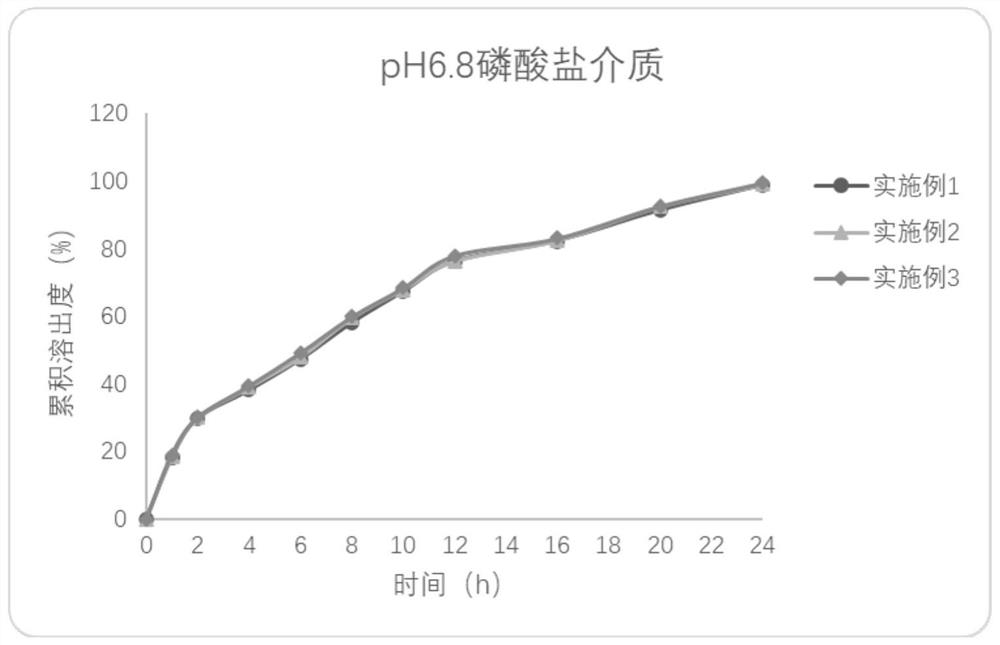
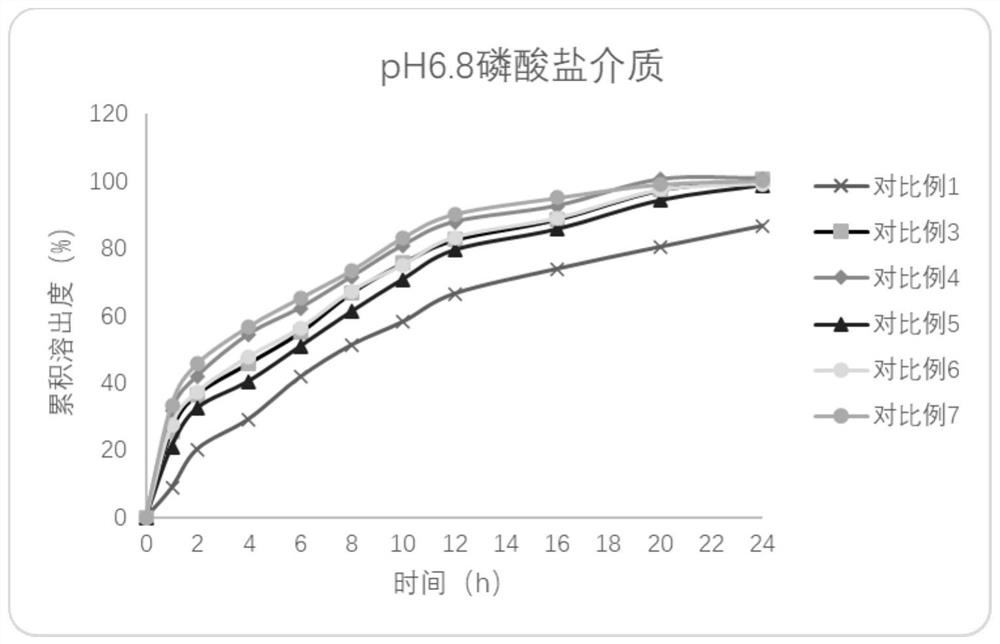
Embodiment 1
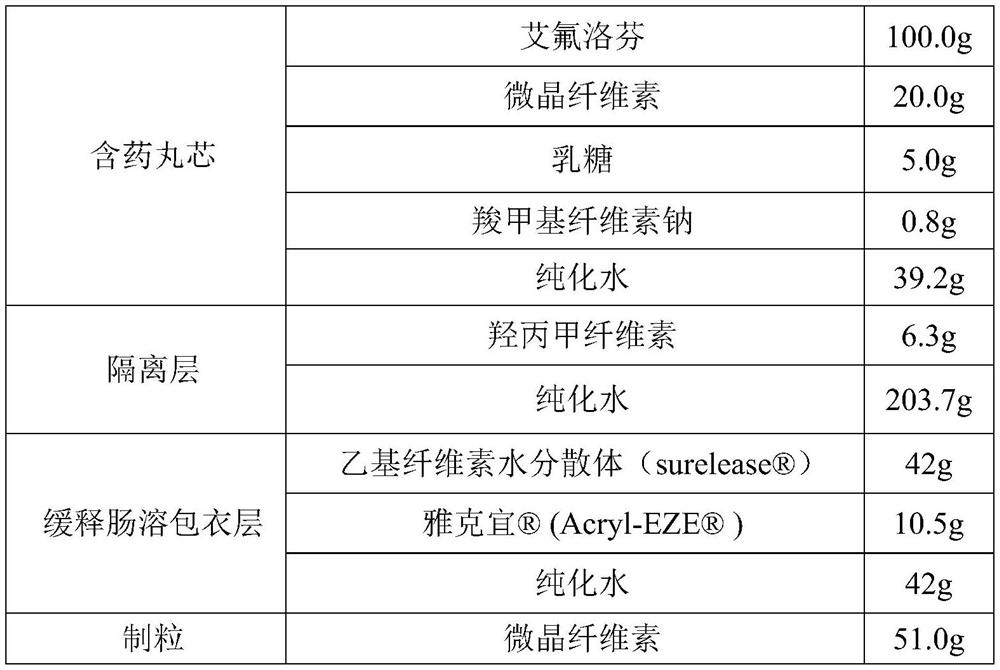
[0045] A kind of Effluprofen slow-release tablet, its tablet formula (1000 preparation unit quantities) is as follows:
[0046]
[0047]
[0048] The preparation method is as follows:
[0049] 1. Preparation of drug-containing pill core:
[0050] 1.1 Pre-treatment: Weigh the raw materials of Effluprofen according to the formula; auxiliary materials: lactose and microcrystalline cellulose, pass through a 60-mesh sieve respectively, and set aside.
[0051] 1.2 Preparation of binder solution: take the formula amount of sodium carboxymethylcellulose and add it to 39.2g of purified water, stir to dissolve, and set aside. The mass content of sodium carboxymethylcellulose in the binder solution is 2.0%.
[0052] 1.3 Preparation of drug-containing pill core: Mix efloprofen, lactose, and microcrystalline cellulose evenly, slowly add binder solution to make soft material, and prepare pellets by extrusion spheronization, and place the prepared pellets in an oven at 60°C Medium-d...
Embodiment 2
[0066] A kind of Effluprofen slow-release tablet, its tablet formula (1000 preparation unit quantities) is as follows:
[0067]
[0068] The preparation method is as follows:
[0069] 1. Preparation of drug-containing pill core:
[0070] 1.1 Pre-treatment: Weigh the raw materials of Effluprofen according to the formula; auxiliary materials: lactose and microcrystalline cellulose, pass through a 60-mesh sieve respectively, and set aside.
[0071] 1.2 Preparation of binder solution: take the formula amount of sodium carboxymethyl cellulose and add 39.2g of purified water, stir to dissolve, and set aside. The mass content of sodium carboxymethylcellulose in the binder solution is 2.0%.
[0072] 1.3 Preparation of drug-containing pill core: Mix efloprofen, lactose, and microcrystalline cellulose evenly, slowly add binder solution to make soft material, and prepare pellets by extrusion spheronization, and place the prepared pellets in an oven at 60°C Medium-dried until the wate...
Embodiment 3
[0086] A kind of Effluprofen slow-release tablet, its tablet formula (1000 preparation unit quantities) is as follows:
[0087]
[0088]
[0089] The preparation method is as follows:
[0090] 1. Preparation of drug-containing pill core:
[0091] 1.1 Pre-treatment: Weigh the raw materials of Effluprofen according to the formula; auxiliary materials: lactose and microcrystalline cellulose, pass through a 60-mesh sieve respectively, and set aside.
[0092] 1.2 Preparation of binder solution: take the formula amount of sodium carboxymethyl cellulose and add 39.2g of purified water, stir to dissolve, and set aside. The mass content of sodium carboxymethylcellulose in the binder solution is 2.0%.
[0093] 1.3 Preparation of drug-containing pill core: Mix efloprofen, lactose, and microcrystalline cellulose evenly, slowly add binder solution to make soft material, and prepare pellets by extrusion spheronization, and place the prepared pellets in an oven at 60°C Medium-dried ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com