Didrogesterone and preparation method thereof
A technology of dydrogesterone and ethyl, applied in the field of dydrogesterone, dydrogesterone and its preparation, can solve the problems of short yield, low yield and lengthy synthesis process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1) Add 126kg of methanol to the dry reaction kettle, pass through 42kg of hydrogen chloride to prepare an acid-alcohol solution, add 20kg of 3,20-bis(ethyldioxy)-9β-10α-pregna-5,7-diene , react at room temperature for 50 minutes; add 40 kg of water to the reaction solution, add 180 kg of ammonia water, add time for 2 hours to crystallize, and grow the crystal for 2 hours, filter and wash to obtain 15 kg of deprotection group intermediate;
[0031] 2) Add 100 kg of toluene to the transposition tank, put in the deprotection group intermediate, stir and add 2.88 kg of acetic acid, heat to reflux for transposition, add 50 kg of water for extraction after the reaction, collect the toluene phase, and evaporate the toluene to dryness;
[0032] 3) Add 193 kg of ethanol, keep stirring for 2.5 hours for crystallization, centrifuge, and dry to obtain the dydrogesterone product with a product yield of 44.5%.
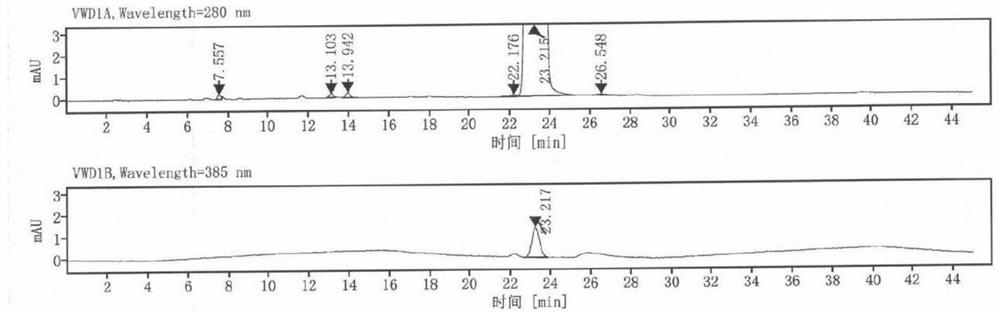
[0033] The dydrogesterone obtained in embodiment 1 is analyzed by a chro...
Embodiment 2
[0040] 1) Add 118.8kg of ethanol to the dry reaction kettle, pass through 29.5kg of hydrogen chloride to prepare an acid-alcohol solution, add 3,20-bis(ethyldioxy)-9β-10α-pregna-5,7-bis 20 kg of alkene was reacted at room temperature for 60 minutes; 35 kg of water was added to the reaction solution, 160 kg of ammonia was added, the feeding time was 2 hours to crystallize, the crystal growth time was 2 hours, filtered, washed, and 13 kg of deprotected intermediate was obtained;
[0041] 2) Add 61.2 kg of toluene to the indexing tank, put in the deprotection intermediate, stir and add 1.8 kg of acetic acid, heat to reflux to remove the protecting group, add 40 kg of water for extraction after the reaction, collect the toluene phase, and evaporate the toluene to dryness;
[0042] 3) Add 177 kg of acetone, keep stirring for 2 hours for crystallization, centrifuge, and dry to obtain dydrogesterone product with a product yield of 45.2%.
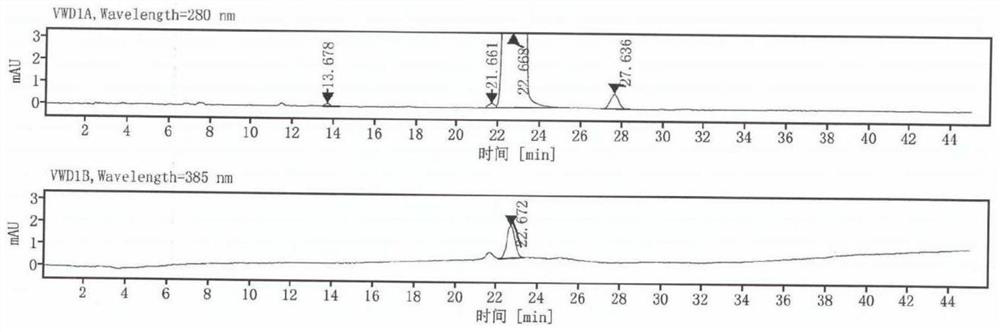
[0043] The dydrogesterone obtained in embodi...
Embodiment 3
[0050] 1) Add 108kg of isopropanol to a dry reaction kettle, pass through 21.6kg of hydrogen chloride to prepare an acid-alcohol solution, add 3,20-bis(ethyldioxy)-9β-10α-pregna-5,7- 20 kg of diene, reacted at room temperature for 70 minutes; 30 kg of water was added to the reaction solution, 120 kg of aqueous ammonia was added, the feeding time was 4 hours to crystallize, the crystal growth time was 1 hour, filtered, washed, and 10 kg of the deprotected intermediate was obtained;
[0051] 2) Add 100 kg of toluene to the transposition tank, put in the deprotected intermediate, stir and add 2.88 kg of acetic acid, heat to reflux for transposition, add 50 kg of water for extraction after the reaction, collect the toluene phase, and evaporate the toluene to dryness;
[0052] 3) Add 177 kg of acetone, keep stirring for 2 hours for crystallization, centrifuge, and dry to obtain the dydrogesterone product with a product yield of 46%.
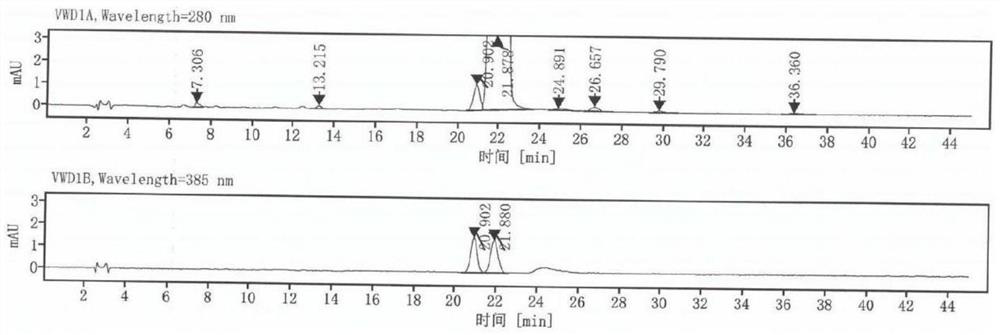
[0053] The dydrogesterone obtained in embodimen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com