Application of aristolochic acid IVa in preparation of antihistamine or pneumonia treatment drugs
A technology of aristolochic acid and antihistamine, which is applied in anti-inflammatory agents, drug combinations, antipyretics, etc., can solve the undiscovered problems of aristolochic acid IVa, etc., achieve significant antihistamine effect and reduce tissue inflammation Exudation, the effect of reducing the amount of inflammation exudation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Effects of AA-IVa on Histamine-Induced Increased Vascular Permeability in Mice
[0036] 1. Test reagent
[0037] Evans blue (EB): Sinopharm Chemical Reagent Co., Ltd., batch number: 20180125; 0.9% sodium chloride injection: Chenxin Pharmaceutical Co., Ltd., batch number: 1801022725; Formamide: Tianjin Damao Chemical Reagent Factory, batch number : 20180416; histamine: Sinopharm Chemical Reagent Co., Ltd., batch number 20160711; AA-IVa (molecular formula: C 17 h 11 NO 8 , with a molecular weight of 293.27), derived from the traditional Chinese medicine Asarum, purchased from Beijing Saibaicao Technology Co., Ltd., batch number: SH18121005.
[0038] 2. Test materials
[0039] Mouse strain: ICR; sex: male.
[0040] Animal weight: 23-25g animals were selected, and the test substance was administered after 1 day of adaptive feeding. Source: Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. Animal requirements: no special pathogenic bacteria.
...
Embodiment 2
[0090] Example 2: AA-IVa alleviates histamine-induced pneumonia
[0091] 1. Experimental method
[0092] (1) The establishment of the model, animal grouping and administration are the same as in Example 1.
[0093] (2) Pathological examination of lung tissue: After the mice were sacrificed by devertebralization, they were dissected and the lung tissue was taken out. The lung tissue was fixed with 10% neutral formalin for later use. The submitted tissue was fully fixed with formaldehyde, dehydrated with ethanol step by step, transparent in xylene, embedded in paraffin, and routinely prepared 3 μm paraffin sections. Stained with HE, Masson, PAS respectively, and optical microscope (DP71 type, OLYMPUS, magnified 400 times) to check the tissue inflammatory exudation.
[0094] 2. Experimental results
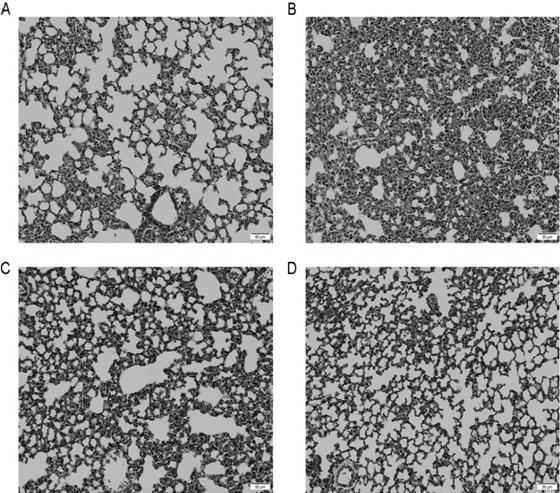
[0095] Lung histopathological results showed: figure 2 A is the normal control group, showing normal structure of alveoli and trachea in lung tissue. figure 2 B is the model ...
Embodiment 3
[0103] Embodiment 3 toxicity test
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com