Difunctional nanorod-like manganese oxide catalyst as well as preparation method and application thereof
A manganese oxide and nano-rod technology, which is applied in the field of nano-rod manganese oxide catalyst and its preparation, can solve the problems of active component vanadium toxicity, human health and environmental hazards, and achieve low price, low cost, and catalytic removal performance Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
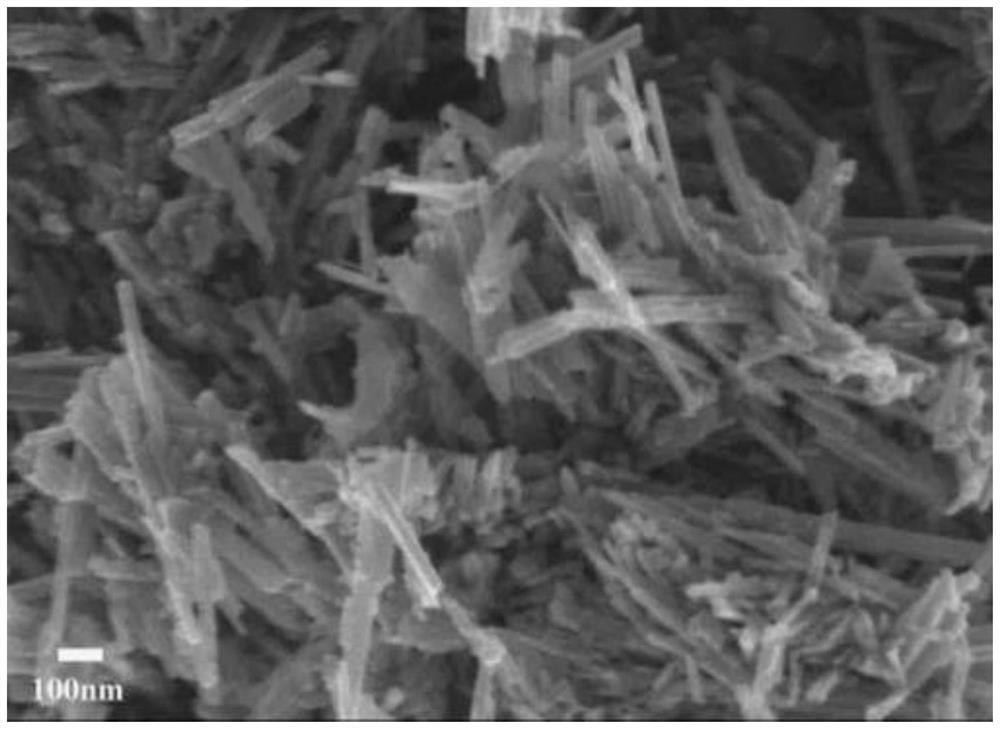
[0025] 0.02mol Mn(CH 3 COO) 2 4H 2 O and 0.02mol KMnO 4 Add it into 160mL distilled water, stir it with a magnetic stirrer for 0.5h to make it fully mixed; pour the evenly mixed liquid into the reaction kettle, and hydrothermally treat it at a temperature of 140°C for 12h; then let it stand at room temperature; filter the mixed liquid, And repeated washing with ethanol and deionized water for 3 times in sequence, after drying the filtered sediment at 80°C for 12h, the dried precipitate (sample) was collected in a crucible and put into a muffle furnace, at 450°C Calcined for 4 hours under temperature conditions; then tabletted, crushed, and sieved to 50 mesh to obtain a nanorod-shaped manganese oxide catalyst with dual functions for removing nitrogen oxides and dichloroethane, denoted as α-MnO x -1 Catalyst.
[0026] α-MnO x The scanning electron microscope picture of -1 catalyst is as follows figure 1 shown, from figure 1 It can be seen that the catalyst prepared in thi...
Embodiment 2
[0028] 0.02mol Mn(CH 3 COO) 2 4H 2 O and 0.02mol KMnO 4 Add it into 160mL distilled water, stir it with a magnetic stirrer for 0.5h to make it fully mixed; pour the evenly mixed liquid into the reaction kettle, and hydrothermally treat it at a temperature of 90°C for 24h; then let it stand at room temperature; filter the mixed liquid, Repeated washing with ethanol and deionized water for 3 times, dried the filtered sediment at 80°C for 12 hours, collected the dried samples into a crucible and put them in a muffle furnace, and calcined them for 4 hours at a temperature of 450°C; Through tableting, crushing, and sieving with 50 meshes, a nanorod-shaped manganese oxide catalyst with dual functions for removing nitrogen oxides and dichloroethane is obtained, which is denoted as α-MnO x -2 Catalyst.
[0029] α-MnO x The scanning electron microscope picture of -2 catalyst is as follows figure 2 shown, from figure 2 It can be seen that the catalyst prepared in this example h...
Embodiment 3
[0035] The catalyst prepared by 0.6g embodiment 1 and embodiment 2 is placed in a fixed bed reactor and carries out HC-SCR catalytic activity evaluation, experimental condition is: NO volume concentration is 800ppm, C 3 h 8 The volume concentration is 600ppm, O 2 Volume concentration is 6.5%, N 2 For balance gas, the total gas flow is 450mL min -1 , airspeed is 19000h -1 , The reaction temperature is 150-550°C.
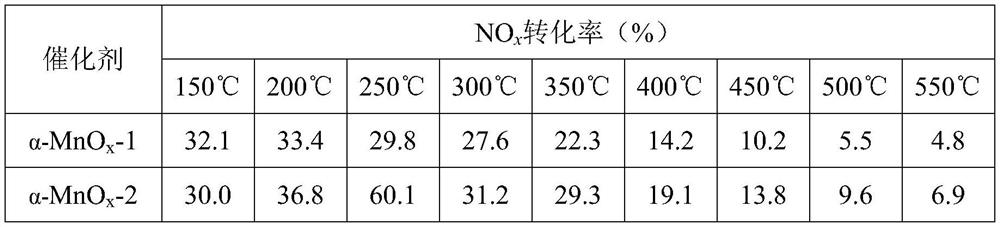
[0036] Detecting NO Using Infrared Gas Analyzers x Concentration, activity of the catalyst at different temperatures, see Table 2.
[0037] The activity of catalyst catalytic reduction of nitrogen oxides under different temperatures when table 2 oxygen content is 6.5%
[0038]
[0039] As can be seen from Table 2, the α-MnO prepared in Example 1 x -1 Catalyst can make NO at 200℃ x Conversion rate reaches 33.4%, the α-MnO that embodiment 2 makes x -2 Catalyst can make NO at 250℃ x The conversion rate reached 60.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com