Resolution method of 3-acetyl-2, 2-dimethyl cyclopropane carboxylic acid
A technology of dimethylcyclopropane carboxylic acid and acetyl group, which is applied in the field of resolution of 3-acetyl-2,2-dimethylcyclopropane carboxylic acid, can solve the problem of light instability, light stability, Problems such as complex production routes and poor safety, etc., achieve the effects of easy operation, high optical purity, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
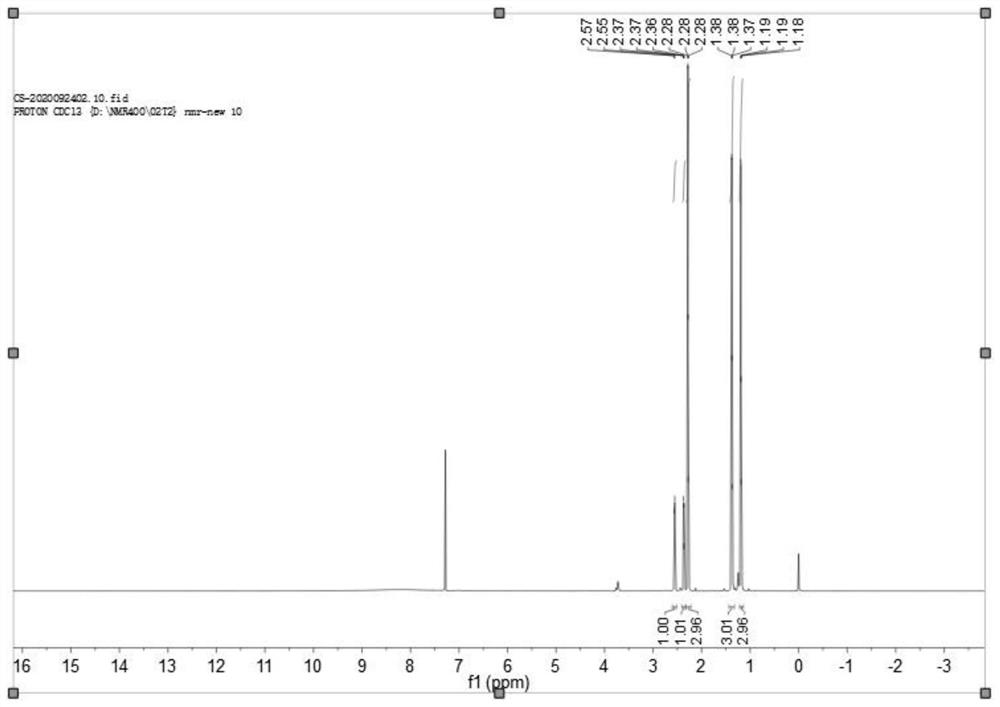
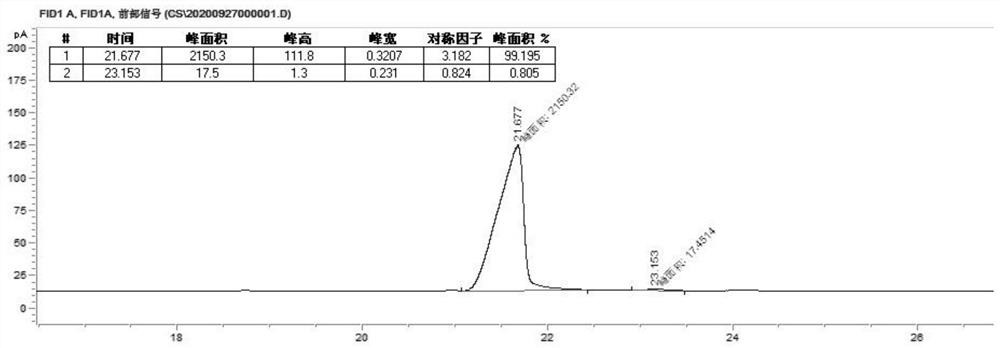
Image
Examples
Embodiment 1
[0031]
[0032] (1) Add 31.2 g of water to the reactor, and then add 15.6 g of 3-acetyl-2,2-dimethylcyclopropanecarboxylic acid.
[0033] (2) In the stirring state, add 25% ammonia water to the reaction kettle, the pH value of the condition system is 7, then start to lower the temperature, and the temperature drops to 10°C.
[0034] (3) When the temperature drops to 10°C, keep warm for the reaction.
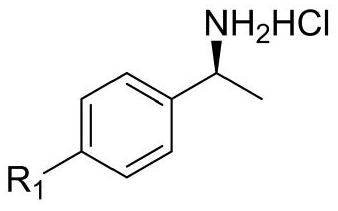
[0035] (4) Add 8.58 g of chiral resolving agent (S)-(-)-1-(p-toluene)ethylamine hydrochloride (2a) into the reaction kettle, then add 8.58 g of water, and dissolve under stirring.
[0036] (5) Slowly add the aqueous solution of the resolving agent in (4) to (3) dropwise, and continue to keep stirring at 10°C for 2 hours after the dropwise addition, there will be Methyl cyclopropane carboxylic acid double salt generation.
[0037] (6) Filter, add dilute acid to the mother liquor to adjust the pH to 3, stir at 10°C for 1 hour, filter, the solid is racemic 3-acetyl-2,2-dimethyl...
Embodiment 2
[0041]
[0042] (1) Add 31.2 g of water to the reactor, and then add 15.6 g of 3-acetyl-2,2-dimethylcyclopropanecarboxylic acid.
[0043] (2) In the stirring state, add 25% ammonia water to the reaction kettle, the pH value of the condition system is 7, then start to lower the temperature, and the temperature drops to 10°C.
[0044] (3) When the temperature drops to 10°C, keep warm for the reaction.
[0045] (4) Add 10.13 g of chiral resolving agent (S)-(+)-Alpha-methyl-4-nitrobenzylamine hydrochloride (2b) into the reaction kettle, then add 10.13 g of water, and dissolve under stirring clear.
[0046] (5) Slowly add the aqueous solution of the resolving agent in (4) to (3) dropwise, and continue to keep stirring at 10°C for 2 hours after the dropwise addition, there will be Methyl cyclopropane carboxylic acid double salt generation.
[0047](6) Filter, add dilute acid to the mother liquor to adjust the pH to 3, stir at 10°C for 1 hour, filter, the solid is racemic 3-ace...
Embodiment 3
[0050]
[0051] (1) Add 31.2 g of water to the reactor, and then add 15.6 g of 3-acetyl-2,2-dimethylcyclopropanecarboxylic acid.
[0052] (2) In the stirring state, add 25% ammonia water to the reaction kettle, the pH value of the condition system is 7, then start to lower the temperature, and the temperature drops to 10°C.
[0053] (3) When the temperature drops to 10°C, keep warm for the reaction.
[0054] (4) Add 8.68 g of chiral resolving agent (S)-4-(1-aminoethyl)phenol hydrochloride (2c) into the reaction kettle, then add 8.68 g of water, and dissolve under stirring.
[0055] (5) Slowly add the aqueous solution of the resolving agent in (4) to (3) dropwise, and continue to keep stirring at 10°C for 2 hours after the dropwise addition, there will be Methyl cyclopropane carboxylic acid double salt generation.
[0056] (6) Filter, add dilute acid to the mother liquor to adjust the pH to 3, stir at 10°C for 1 hour, filter, the solid is racemic 3-acetyl-2,2-dimethylcyclo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com