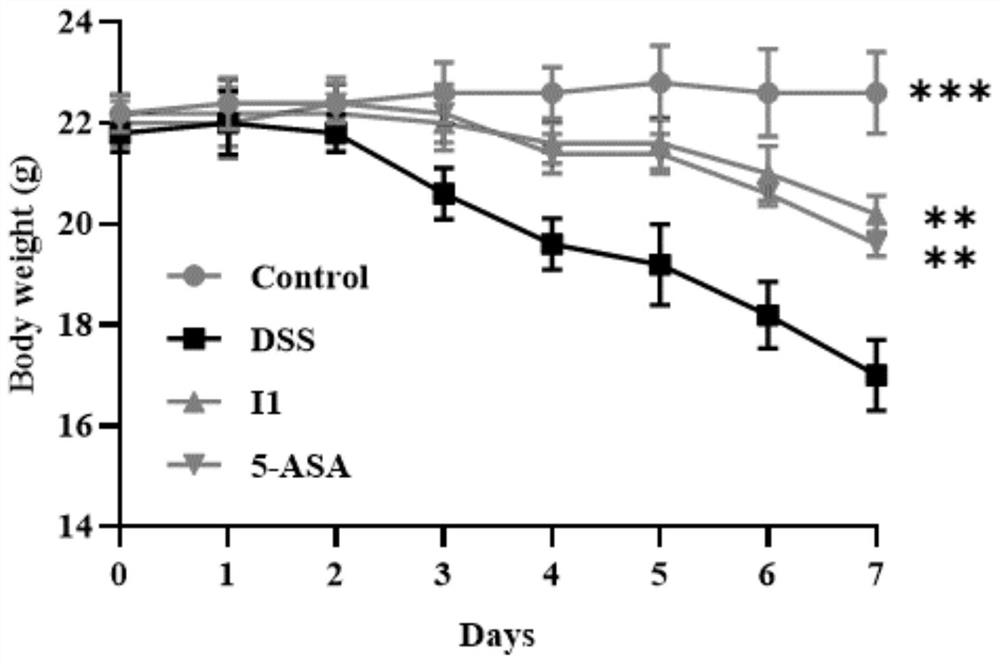
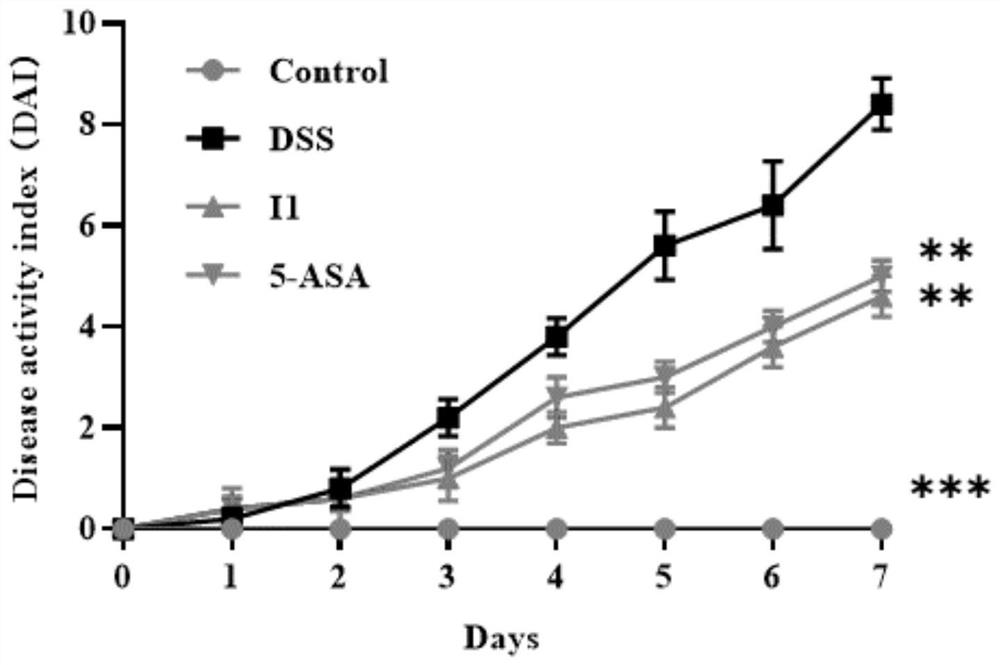
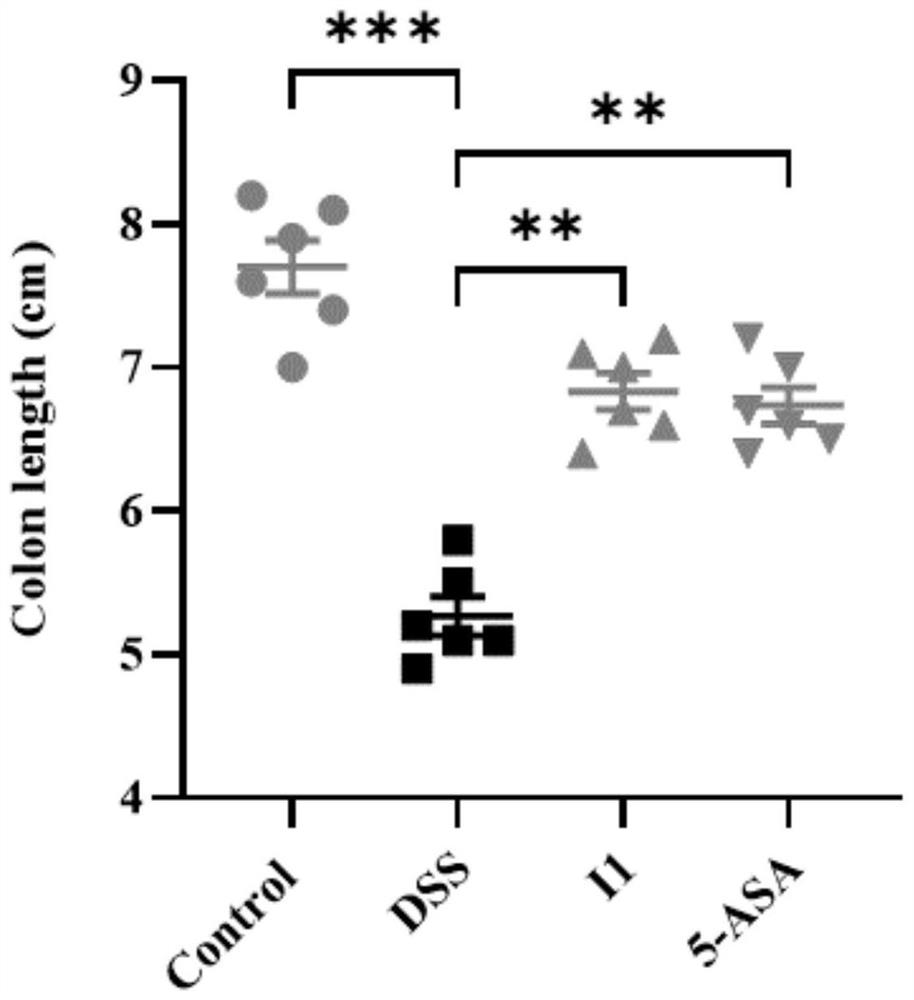
Benzoxazole compound as well as preparation method, pharmaceutical composition and application thereof
A technology for benzoxazole and compounds, which is applied in the field of benzoxazole compounds and their preparation, can solve the problems of low bioavailability and poor stability, and achieve the effect of simple and easy preparation method and excellent therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Synthesis of 2-(1-(tert-butyl)-5-(furan-2-yl)-1H-pyrazol-3-yl)benzo[d]oxazole
[0045]
[0046] Place 2.8g of potassium tert-butoxide in 11mL of THF to form a suspension, and cool in an ice bath at 0°C; dissolve 1.1g of acetylfuran and 2.7mL of diethyl oxalate in 11mL of ethylene glycol dimethyl ether, and dissolve this The solution was added dropwise to the suspension of potassium tert-butoxide at 0°C; the mixture was stirred at room temperature for 1 hour. After all the acetylfuran was consumed as monitored by TLC, 7.5 mL of 1M HCl solution was added. The crude product was extracted with ethyl acetate, the organic phase was dried over anhydrous sodium sulfate, spin-dried under reduced pressure, and purified by silica gel column chromatography, eluting with petroleum ether: ethyl acetate (4:1) to obtain 4-(furan-2 -yl)-2,4-dioxobutanoic acid ethyl ester.
[0047] 1.4 g of ethyl 4-(furan-2-yl)-2,4-dioxobutyrate was dissolved in ethanol, 0.9 g of tert-but...
Embodiment 2
[0054] Example 2: Synthesis of 2-(1-(tert-butyl)-5-(furan-2-yl)-1H-pyrazol-3-yl)-5-methylbenzo[d]oxazole
[0055]
[0056] Using o-amino-p-cresol as a raw material, see Example 1 for the synthesis method.
[0057] The NMR data are as follows:
[0058] 1 H NMR (400MHz, DMSO-d6) δ7.92(s, 1H), 7.66(d, J=8.3Hz, 1H), 7.57(s, 1H), 7.23(d, J=8.3Hz, 1H), 7.15 (s,1H),6.80(d,J=3.3Hz,1H),6.68(d,J=2.6Hz,1H),2.44(s,3H),1.53(s,9H).
[0059] 13 C NMR(101MHz,DMSO-d6)δ158.21,148.51,144.48,142.95,141.90, 137.33,134.68,134.21,126.86,119.96,113.31,112.25,112.12,110.82,62.76,40.62, 40.42,40.21,40.00,39.79,39.58 ,39.37,34.94,31.60,30.28,29.96,21.46.
Embodiment 3
[0060] Example 3: Synthesis of 2-(1-(tert-butyl)-5-(furan-2-yl)-1H-pyrazol-3-yl)-5-chlorobenzo[d]oxazole
[0061]
[0062] Using 4-chloro-2-aminophenol as raw material, see Example 1 for the synthesis method.
[0063] The NMR data are as follows:
[0064] 1 H NMR (400MHz, DMSO-d6) δ8.07 (dd, J = 7.5, 1.6Hz, 1H), 7.84–7.72 (m, 2H), 7.40 (dd, J = 7.5, 1.5Hz, 1H), 7.27– 7.16(m,2H),6.98(t,J=7.5Hz,1H),1.46(s,10H).
[0065] 13 C NMR (101MHz, DMSO-d6) δ158.07, 152.16, 150.75, 142.66, 140.52, 131.95, 131.20, 130.80, 126.55, 119.50, 112.14, 111.51, 104.50, 60.49, 28.94.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com