Method for synthesizing isophorone diisocyanate
A technology of isophorone diisocyanate and isophorone dicarbamate, which is applied in the field of isocyanate synthesis, can solve the problems of difficult catalyst separation, high boiling point of by-products, and great potential safety hazards, and achieves good industrial application prospects, Mild reaction conditions and little safety hazard
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
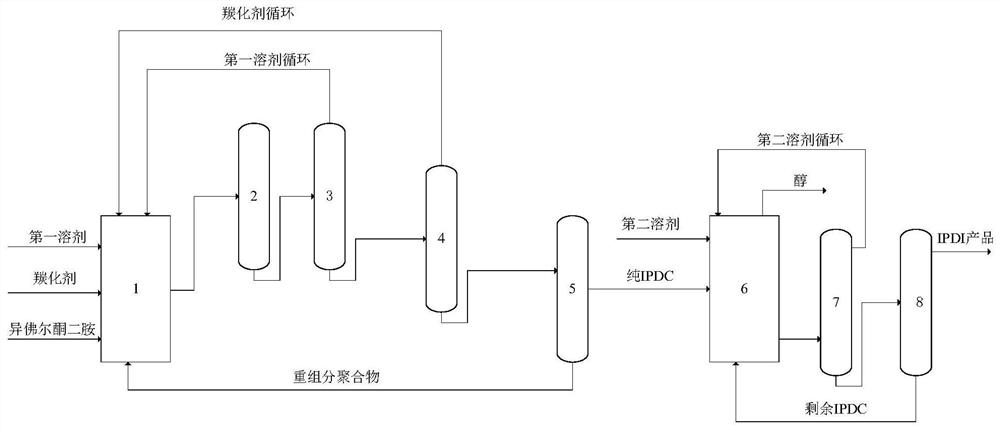
[0051] This embodiment provides a method for synthesizing isophorone diisocyanate, the synthesis system used in the method is as follows figure 1 As shown, the method includes the following steps:
[0052] (1) Mix isophorone diamine, urea, ethanol and iron oxide catalyst at 190°C for carbonylation for 5 hours, separate solid from liquid and perform purification treatment to obtain isophorone dicarbamate;
[0053] The mol ratio of described urea and described isophorone diamine is 2.1:1, and the mol ratio of described ethanol and described isophorone diamine is 20:1, and the quality of iron oxide catalyst is described isophorone diamine. 10% of the mass of ketone diamine;
[0054] The purification treatment includes deamination treatment, ethanol removal treatment and urea removal treatment of the filtrate obtained by the solid-liquid separation; the ethanol, urea and heavy component polymer obtained by the purification treatment are returned to carry out carbonylation reactio...
Embodiment 2
[0060] The present embodiment provides a kind of method for synthesizing isophorone diisocyanate, described method comprises the following steps:
[0061] (1) Mix isophoronediamine, n-propyl carbamate, n-propanol and titanium dioxide catalyst at 200°C for carbonylation reaction for 5 hours, separate solid and liquid and perform purification treatment to obtain isophorone dicarbamate ester;
[0062] The mol ratio of described n-propyl carbamate and described isophorone diamine is 15:1, and the mol ratio of described n-propanol and described isophorone diamine is 20:1, and the mol ratio of titanium oxide catalyst The mass is 10% of the mass of the isophorone diamine;
[0063] The purification treatment includes deamination treatment, de-n-propanol treatment and decarbamate treatment of the filtrate obtained by the solid-liquid separation; the n-propanol, decarbamate n-propyl ester and The heavy fraction polymer returns to the carbonylation reaction;
[0064] (2) Mix the isoph...
Embodiment 3
[0069] The present embodiment provides a kind of method for synthesizing isophorone diisocyanate, described method comprises the following steps:
[0070] (1) Mix isophorone diamine, methyl carbamate, methanol, zirconium oxide and dicobalt trioxide catalyst for carbonylation reaction at 210°C for 5 hours, separate solid and liquid and carry out purification treatment to obtain isophorone di carbamate;
[0071] The mol ratio of described methyl carbamate and described isophorone diamine is 5:1, and the mol ratio of described methyl alcohol and described isophorone diamine is 50:1, and the mass of cobalt trioxide catalyst It is 5% of the mass of the isophorone diamine, and the mass of the zirconia catalyst is 5% of the mass of the isophorone diamine;
[0072] The purification treatment includes deamination treatment, demethanol treatment and decarbamate treatment of the filtrate obtained by the solid-liquid separation; the methanol, decarbamate methyl ester and heavy component ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com