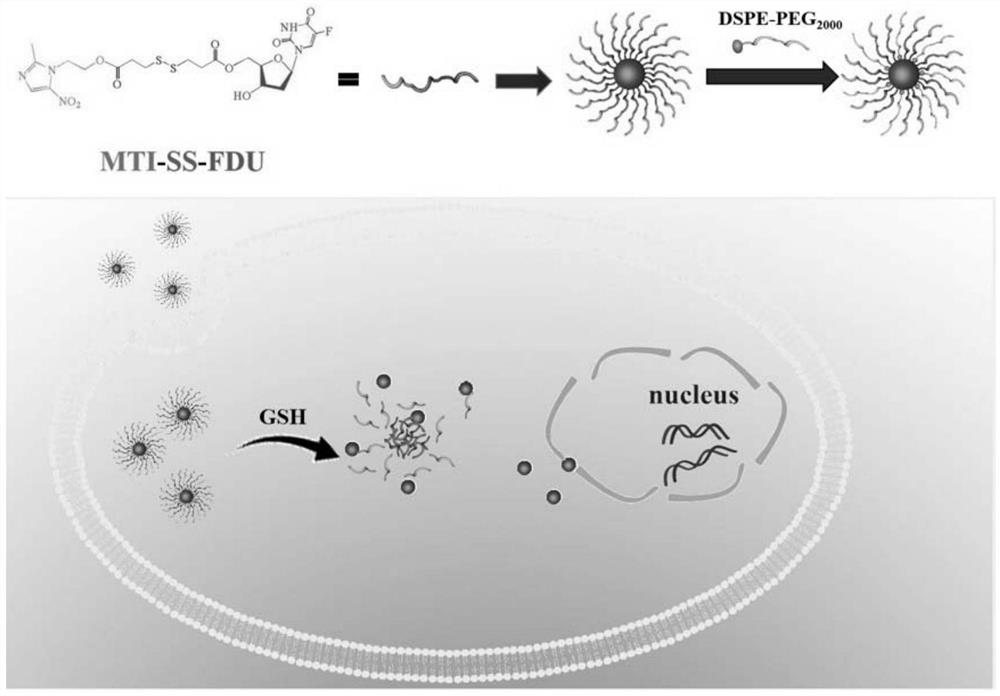
Targeted tumor microenvironment amphiphilic micromolecule nano-drug and preparation method thereof
A nano-drug delivery system and compound technology, applied in the field of amphiphilic small molecule nano-drugs and their preparation, can solve problems such as poor solubility, low bioavailability, and lack of selectivity of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
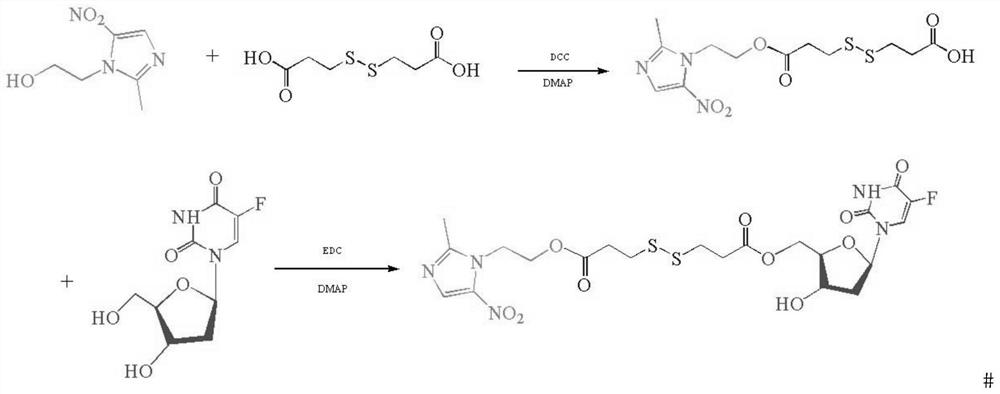
[0098] Embodiment 1, the synthesis of metronidazole-fluridine
[0099] according to figure 2 The flowchart shown in the synthesis
[0100] Weigh metronidazole (MTI, 684mg, 4mmol) into a 250mL round bottom flask, measure 80mL of anhydrous dichloromethane (DCM) into the reaction flask, turn on magnetic stirring, add 3,3' -Dithiodipropionic acid (DTPA, 1260mg, 6mmol), 4-dimethylaminopyridine (DMAP, 171mg, 1.4mmol), after 5min in ice bath, weigh dicyclohexylcarbodiimide (DCC, 989mg, 4.8 mmol) was added into dichloromethane, and reacted at room temperature for 24h. After the reaction, the white precipitate 1,3-dicyclohexylurea (DCU) precipitated in the solvent was removed by filtration through a Buchner funnel, and the solvent DCM was evaporated to dryness using a rotary evaporator at 30°C. Using methanol: dichloromethane (volume ratio 1:30) as the developing solvent, separated and purified by silica gel column chromatography, and evaporated the solvent to obtain 494 mg of the ...
Embodiment 2
[0120] Embodiment 2, preparation of metronidazole-fluridine nanoparticles
[0121] Metronidazole-fluridine was made into nanoparticles by film hydration method. Weigh 5 mg of MTI-FDU into a flask, and dissolve the MTI-FDU compound with 5 mL of dichloromethane (DCM). Then weigh 20mg of DSPE-PEG2000 into a 15mL centrifuge tube, dissolve DSPE-PEG2000 with 6mL of dichloromethane, slowly drop into the flask, and stir magnetically to make it evenly mixed. DCM was evaporated to dryness by rotary evaporation at 30°C, and a light white film was covered on the bottle wall. A total of 5ml of deionized water was added dropwise, and the flask was placed in a constant temperature water bath at 30°C to incubate for 10min.
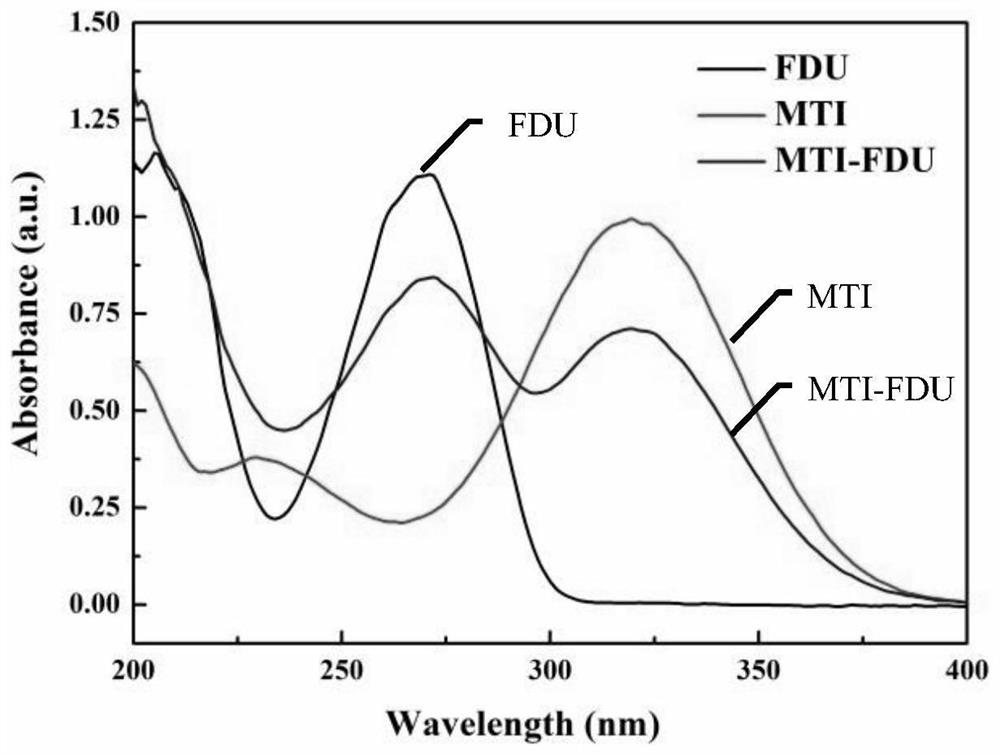
[0122] Characterization of Metronidazole-Foxuridine Nanoparticles
[0123] (1) Transmission electron microscope (TEM) observation
[0124] Dilute the prepared MTI-FDU nano-preparation to an appropriate multiple, drop it on a 200-mesh copper grid, wait for the water to...
Embodiment 3
[0131] Example 3, Preparation of MTI-FDU@Cy5.5 NPs
[0132] Accurately weigh 5mg of MTI-FDU into a round bottom flask, and dissolve the compound with 5mL of dichloromethane. Weigh 20mg of DSPE-PEG2000-Cy5.5 in a 15mL centrifuge tube under dark conditions, dissolve DSPE-PEG2000-Cy5.5 with 6mL of dichloromethane, slowly drop into the flask, and stir magnetically to make it evenly mixed. DCM was evaporated to dryness under reduced pressure at 30°C, and a layer of turquoise film was covered on the bottle wall. A total of 5ml of deionized water was added dropwise, and the flask was placed in a constant temperature water bath at 30°C to incubate for 10min.
[0133] The characterization results of MTI-FDU@Cy5.5 NPs are shown in Example 5.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ld50 | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com