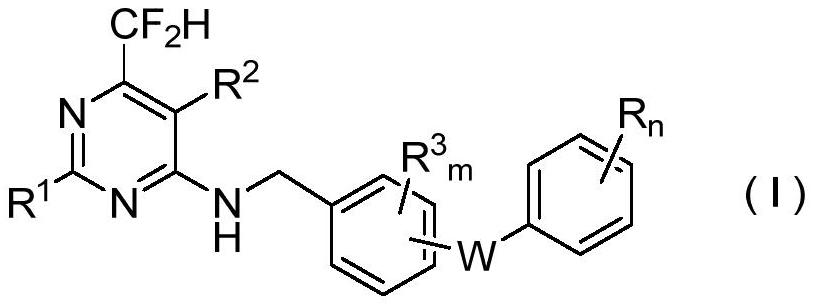
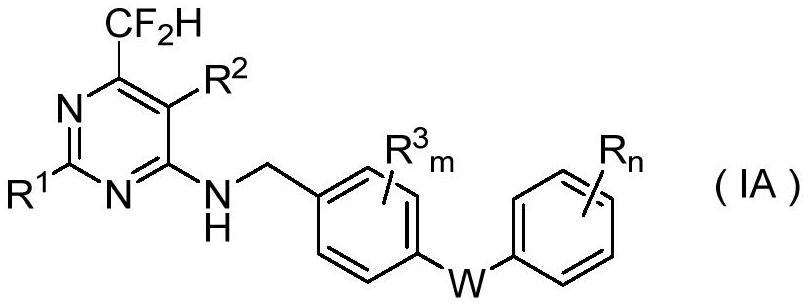
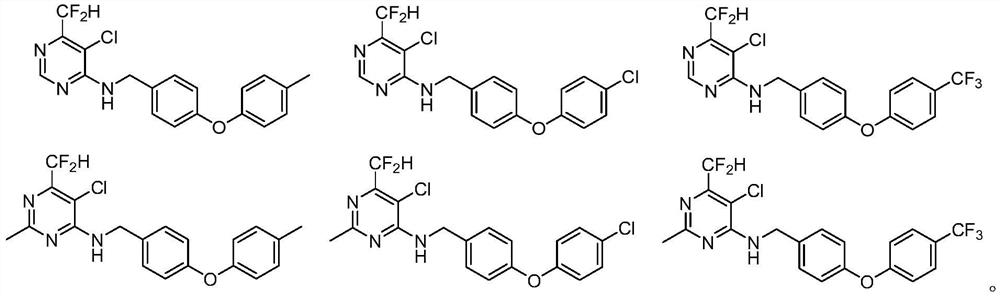
N-aryloxy/thiobenzyl difluoromethyl pyrilamine compound as well as preparation method and application thereof
A technology of thiobenzyldifluoromethylpyrimidinamine and compounds, which is applied in the fields of botany equipment and methods, applications, organic chemistry, etc., can solve the problem that the bactericidal activity of the compound has not been reported, and the insecticidal and acaricidal activities are far inferior to others. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Example 1 This example illustrates the preparation method of compound 02 in Table 1
[0091]
[0092]
[0093] 4-(4-methylphenoxy)benzonitrile p-cresol (0.10mol), 50% aqueous potassium hydroxide solution (11.20g) and toluene (100mL) were heated under stirring to remove toluene and water to obtain 4- Methylphenol potassium salt. Add N,N-dimethylformamide (100 mL), p-bromobenzonitrile (0.10 mol) and cuprous bromide (0.012 mol), and react under stirring and reflux for 2-5 h until the reaction is complete. The reaction solution was slowly poured into ice water with stirring, and the resulting solid was filtered and recrystallized with 95% ethanol to obtain 15.60 g of the title compound as an off-white solid.
[0094] 4-(4-methylphenoxy)benzylamine under nitrogen protection, 4-(4-methylphenoxy)benzonitrile (0.06mol), tetrahydrofuran (150mL) and sodium borohydride (0.12mol), in Under the condition of stirring and reflux, react for 6-20h until the reaction is complete....
Embodiment 2
[0099] Embodiment 2 This embodiment illustrates the preparation method of compound 83 in Table 1
[0100]
[0101]Add sodium methoxide dropwise to a solution of acetamidine hydrochloride (0.33mol) and methanol (50mL) at 5-10°C with stirring (0.63mol) in 30% methanol solution, continue to stir for 2-3h after dropping, add 2-chloro-3-oxo-4,4-difluorobutanoic acid ethyl ester dropwise, let it warm up to room temperature to complete the reaction. After methanol was removed, ethyl acetate was added, insoluble matter was filtered off, and the filtrate was concentrated to obtain 32.90 g of the title product as a yellow solid, which was directly used in the next reaction.
[0102] 4,5-dichloro-2-methyl-6-difluoromethylpyrimidine at 0~5℃ under the condition of stirring, 5-chloro-2-methyl-6-difluoromethylpyrimidin-4-ol (0.15mol) was added in batches to phosphorus oxychloride (0.35mol), and after complete dissolution, triethylamine (0.17mol) was added dropwise. Naturally warm up to ...
Embodiment 3
[0105] Example 3 Preparation of 5-chloro-6-difluoromethyl-N-(4-(4-methylphenoxy)benzyl)pyrimidin-4-amine (02) 10% emulsifiable concentrate
[0106] Take an appropriate amount (10% by weight) of the compound of formula (I) provided by the invention such as compound 02 in Table 1, an appropriate amount of cosolvent (such as ethyl acetate or acetone), an appropriate amount of pesticide auxiliary agent and solvent (such as Toluene) etc. into the reactor, first add a certain amount of solvent (such as toluene) and defoamer and stir for 10-30 minutes, then add an appropriate amount of stabilizer, synergist, penetrating agent and other components, continue stirring for 10-30 minutes , adjust the pH value, then put an effective amount of solvent into the kettle, stir evenly and discharge to get 5-chloro-6-difluoromethyl-N-(4-(4-methylphenoxy)benzyl) Pyrimidin-4-amine (02) 10% EC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com