Phosphorylated Tau pT217 protein monoclonal antibody, ELISA kit and application of phosphorylated Tau pT217 protein monoclonal antibody
A monoclonal antibody and polyclonal antibody technology, applied in the field of biomedicine, can solve the problems of low detection sensitivity and low specificity, and achieve the effect of wide detection range, strong specificity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 immune complex preparation
[0036]1) Synthesis of antigen sequences of 217-position threonine (T217) phosphorylated antigens containing tau proteins by solid phase method (amino acid sequences at positions 213 to 227 in tau proteins: PSLPT-pPPTREPKKVA, as shown in SEQ ID NO.5) and antigen sequences containing amino acids at positions 412 to 434 of tau protein (amino acid sequences at positions 412 to 434 in tau proteins: SSTGSIDMVDSPSPlatLADEVSA, As shown in SEQ ID NO.6), performed on ABI's Peptide Synthesizer (431A) in the United States, using Fmoc (9-fluorenemethoxycarbonyl) protocol, the synthesis steps are carried out in accordance with ABI's Polypeptide Synthesis Operation Manual. Purified by high performance liquid chromatography, sequence identification was prepared by mass spectrometry to obtain a phosphorylated antigen sequence containing tau protein 217-position threonine (T217) and an antigen sequence containing tau protein 412~434 amino acids.
[0037...
Embodiment 2
[0039] Example 2 Preparation of monoclonal antibodies
[0040] 2.1 Animal immunity
[0041] Immunized 4 to 6 weeks old BALB / c mice with 217-bit threonine (T217) phosphorylated antigen sequence immune complex containing tau protein (prepared in Example 1) after mixing emulsification with adjuvant, a total of 5 mice, the immunity amount is 0.3 mg / only / time, the immunization mode is abdominal subcutaneous injection, the specific operation steps are shown in Table 1 below:
[0042] Table 1 BALB / c mouse immune processes
[0043] Number of immunizations Immunization time (d) Immune dose Immune pathway First immunization 1 300 μg / pc (equal amount of complete Freund's adjuvant emulsification) Intradermal injection Second immunization 15 300 μg / only (equal amount of incomplete Freud's adjuvant emulsion) subcutaneous injection Third immunization 29 300 μg / only (equal amount of incomplete Freud's adjuvant emulsion) subcutaneous injection Fourth immuni...
Embodiment 3
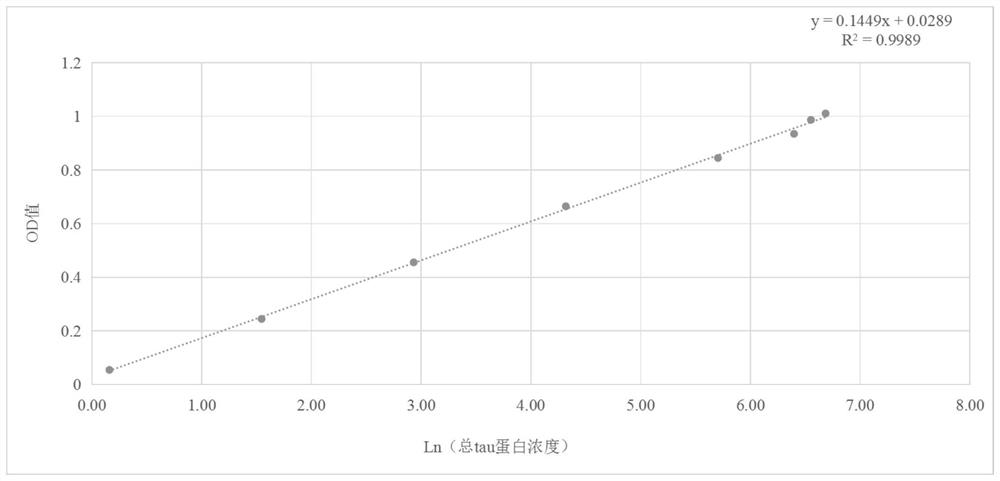
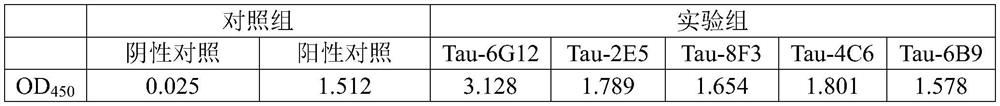
[0078] Example 3 Identification of monoclonal antibodies
[0079] 3.1 Sequence determination of monoclonal antibody secreted by Tau-6G12 of hybridoma cell line
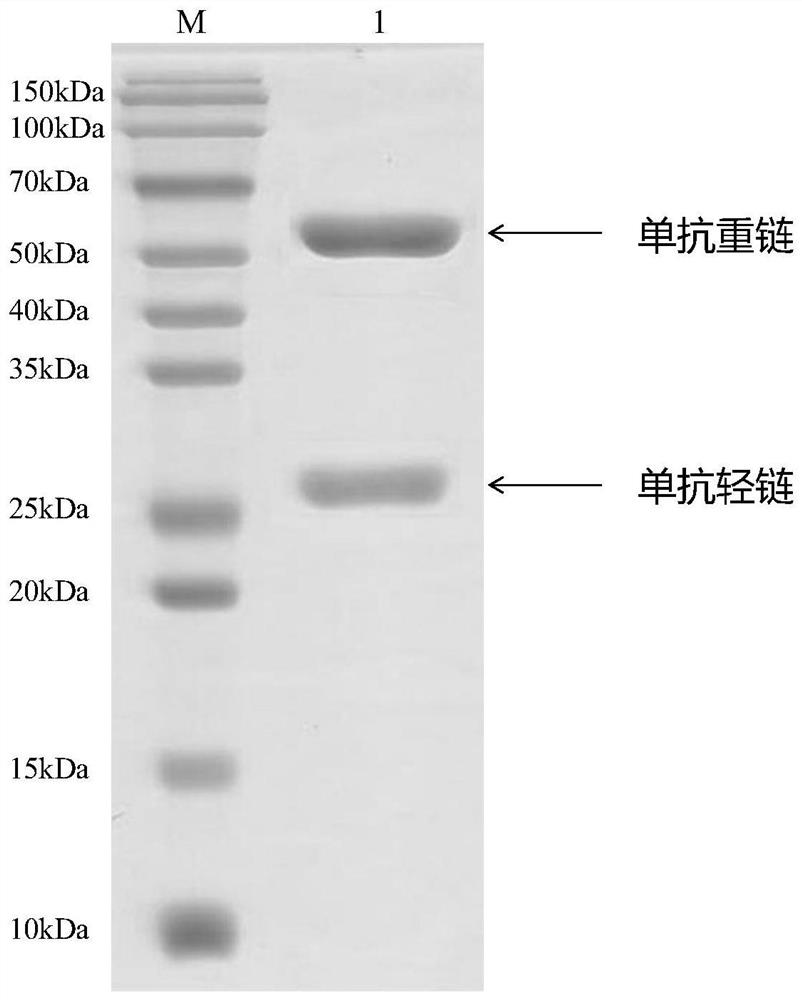
[0080] The total RNA of the hybridoma cell line Tau-6G12 was extracted, the cDNA was reversed with a universal primer, and then the light and heavy chains of the antibody were amplified, the light and heavy chains were separated, cloned to the standard cloning vector for expression, the single colony PCR identified the light chain and heavy chain, and 5 to 10 single colonies with the correct length of light and heavy chains were selected for sequencing, of which 5 sequencing results were almost the same, which was considered to be the true sequence of the antibody. (The sequencing process and a large amount of antibody expression were entrusted to Pujian Biological (Wuhan) Technology Co., Ltd.)
[0081]The monoclonal antibody heavy chain coding gene sequence secreted by hybridoma cell line Tau-6G12 is 1380bp long, as show...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dilution degree | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com