Preparation method and application of cyclic quaternary ammonium salt electrolyte
A quaternary ammonium salt and electrolyte technology, applied in the directions of hybrid capacitor electrolytes, organic chemistry, etc., can solve the problems of unfavorable recycling and reuse, high cost of raw materials and equipment, rare reaction raw materials, etc., and avoid the operation and process of solvent distillation and separation. Simple, high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
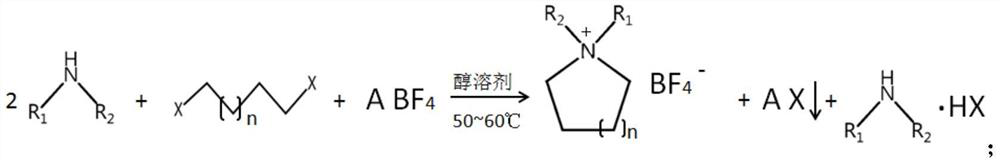
[0047] Preparation of N,N-dimethylpyrrolidinium tetrafluoroborate
[0048] (1), in reactor, adding successively mass concentration is 30% dimethylamine methanol solution (dimethylamine charging capacity is 2mol), 1mol 1,4-dibromobutane and 1mol potassium tetrafluoroborate, 50~60 React at ℃ for 6h to obtain N,N-dimethylpyrrolidinium tetrafluoroborate reaction solution;
[0049] (2) The above reaction solution was filtered to obtain a methanol solution of N,N-dimethylpyrrolidinium tetrafluoroborate, the solution was crystallized at -10°C to produce a large number of white crystals, and then filtered to obtain a high-purity ring quaternary ammonium salt crystals, and finally vacuum-dried at 120 °C for 20 h to obtain high-purity N,N-dimethylpyrrolidinium tetrafluoroborate.
[0050] The purity of the product analyzed by ion chromatography was 99.9%, and the yield (calculated as dihaloalkane) reached 92%;
[0051] Detected by ICP: Na + : 0.77ppm, K + : 0.45ppm, Fe 3+ : 0.2ppm, Ca...
Embodiment 2
[0053] Preparation of N,N-Dimethylpiperidinium tetrafluoroborate
[0054] (1), successively add 30% dimethylamine methanol solution (the dimethylamine feeding amount is 2mol), 1mol 1,5-dichloropentane and 1mol sodium tetrafluoroborate in the reactor, react for 6h at 50~60°C , to obtain N,N-dimethylpiperidine alkynium tetrafluoroborate reaction solution;
[0055] (2) above-mentioned reaction solution is filtered to obtain the ethanolic solution of N,N-dimethylpiperidine alkynium tetrafluoroborate, the solution is crystallized under -10 ℃ condition, produces a large number of white crystals, and then filtered to obtain high-purity The cyclic quaternary ammonium salt crystals are finally vacuum dried at 120°C for 20 hours to obtain high-purity N,N-dimethylpiperidine alkynium tetrafluoroborate.
[0056] The purity of the product analyzed by ion chromatography was 99.9%, and the yield (calculated as dihaloalkane) reached 91%;
[0057] Detected by ICP: Na + : 1.97ppm, K + : 0.54...
Embodiment 3
[0060] Preparation of N,N-diethylpiperidinium tetrafluoroborate
[0061] (1), add 30% concentration of diethylamine ethanol solution (diethylamine feeding amount 2mol), 1mol 1,5-dibromopentane and 1mol ammonium tetrafluoroborate successively in the reaction kettle, react 6h at 50~60 ℃ , to obtain N,N-diethylpiperidine alkynium tetrafluoroborate reaction solution;
[0062] (2) filtering the above reaction solution to obtain an ethanolic solution of N,N-diethyl piperidinium tetrafluoroborate, the solution is crystallized at -10°C to produce a large number of white crystals, and then filtered to obtain high-purity The cyclic quaternary ammonium salt crystal is finally vacuum dried at 120°C for 20h to obtain high-purity N,N-diethylpiperidine alkynium tetrafluoroborate.
[0063] The purity of the product analyzed by ion chromatography was 99.9%, and the yield (calculated as dihaloalkane) reached 87%;
[0064] Detected by ICP: Na + : 0.43ppm, K + : 0.13ppm, Fe 3+ : 0.46ppm, Ca ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com