MQ type silicon resin capable of being quickly cross-linked and cured as well as preparation method and application of MQ type silicon resin
A MQ silicone resin, cross-linking and curing technology, applied in the field of polymer chemistry, to achieve the effect of good application prospects and good structural stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
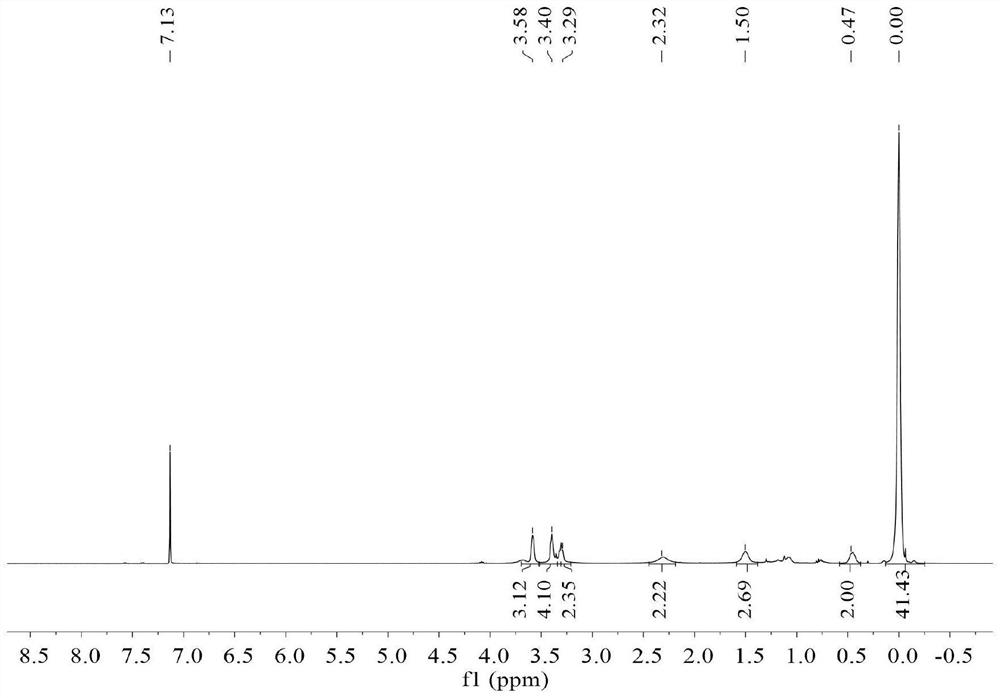
[0079] dry and filled with N 2 In the there-necked flask, add [2-(allyloxy)] ethoxytrimethylsilane of 6.97g (0.0384mol), 20mL toluene and 0.1109g Pt content are the Karstedt catalyst of 2wt%, turn on stirring, at 70 Activated at ℃ for 30 min. Dissolve 20.0 g of commercially available hydrogen-containing MQ resin with a hydrogen content of 0.16 wt% and a viscosity of 4310 cP at 25°C (containing Si-H 0.032 mol) in 20 mL of toluene, and then pass the dissolved clear solution through a syringe pump within 2 hours. It was added dropwise to the reaction system at a uniform rate. After the addition of the materials, the reaction was continued at 70° C. for 3 hours, and the solvent and low boilers were removed to obtain 25.69 g of trimethylsiloxyethoxypropyl-terminated MQ resin.
[0080] The obtained trimethylsiloxyethoxypropyl-terminated MQ resin was mixed with 48.06g (1.5mol) methanol and 1.08g (0.018mol) acetic acid, and the hydroxyl group was deprotected to remove the trimethyls...
Embodiment 2
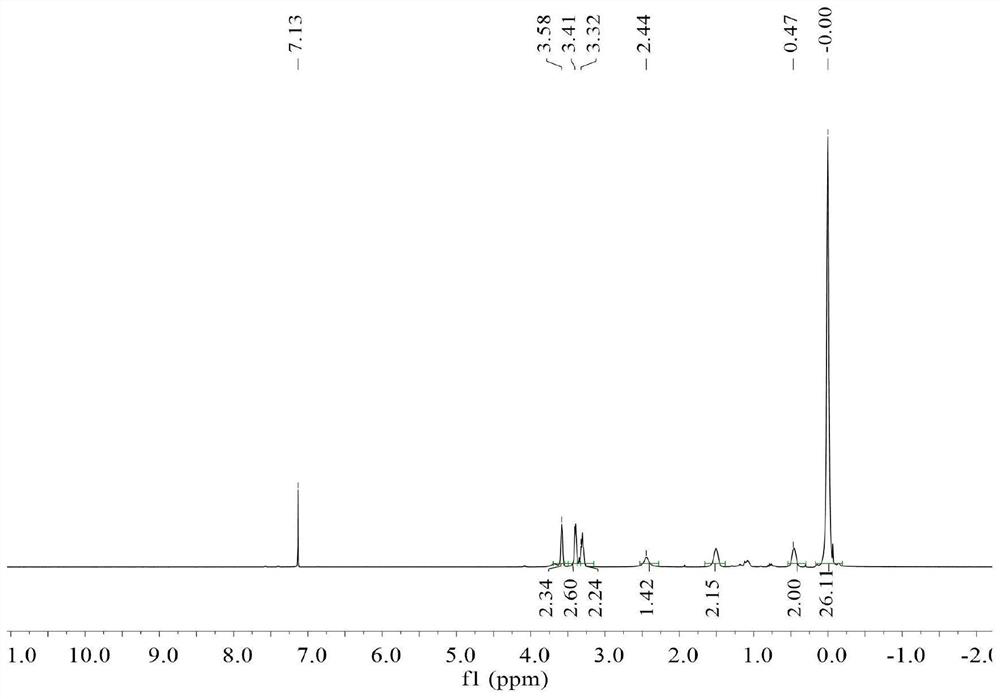
[0082] dry and filled with N 2 In the three-necked flask, add 13.76g (0.075mol) of [2-(allyloxy)]ethoxytrimethylsilane, 20mL of toluene and 0.1733g of Pt content are the Karstedt catalyst of 2wt%, start stirring, at 70 Activated at ℃ for 30 min. Dissolve 20.0 g of commercially available hydrogen-containing MQ resin with a hydrogen content of 0.25 wt% and a viscosity of 2000 cP at 25°C (containing Si-H 0.050 mol) in 20 mL of toluene, and then pass the dissolved clear solution through a syringe pump within 2 hours. It was added dropwise to the reaction system at a uniform rate. After the addition of the materials, the reaction was carried out at 70° C. for 3 hours, and the solvent and low boilers were removed to obtain 25.30 g of trimethylsiloxyethoxypropyl end-capped MQ resin.
[0083] The obtained trimethylsiloxyethoxypropyl terminated MQ resin was mixed with 80.1 g (2.5 mol) methanol and 1.80 g (0.03 mol) acetic acid, and then the hydroxyl group was deprotected to remove th...
Embodiment 3
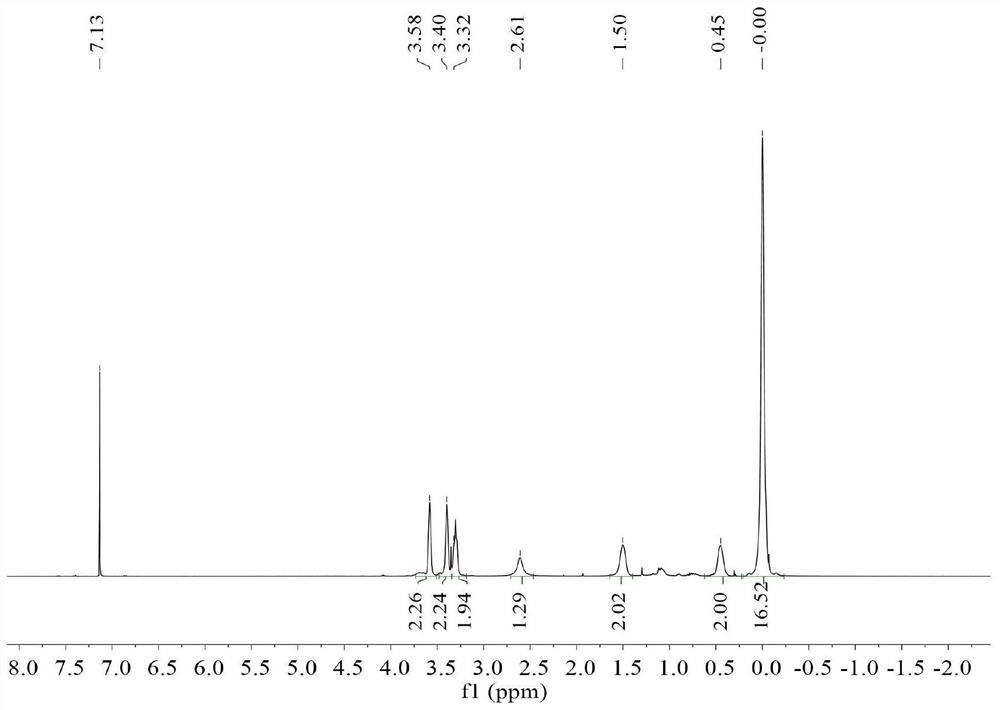
[0085] dry and filled with N 2 In the three-necked flask, add 18.30g (0.105mol) of [2-(allyloxy)]ethoxytrimethylsilane, 20mL of toluene and 0.6700g of Pt content as the Karstedt catalyst of 2wt%, start stirring, at 70 Activated at ℃ for 30 min. Dissolve 20.0 g of commercially available hydrogen-containing MQ resin with a hydrogen content of 0.35 wt% and a viscosity of 375 cP at 25°C (containing Si-H 0.070 mol) in 20 mL of toluene, and then pass the dissolved clear solution through a syringe pump within 2 hours. It was added dropwise to the reaction system at a uniform rate. After the addition of materials, the reaction was carried out at 70° C. for 3 hours, and the solvent and low boilers were removed to obtain 28.53 g of trimethylsiloxyethoxypropyl-terminated MQ resin.
[0086] The obtained trimethylsiloxyethoxypropyl end-capped MQ resin was mixed with 99.0g (3.1mol) methanol and 2.2g (0.037mol) acetic acid, and the hydroxyl group was deprotected to remove the trimethylsily...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com