Drug-loaded nano-particles for hepatic artery chemoembolization and preparation method of drug-loaded nano-particles
A technology of drug-loaded nanoparticles and nanoparticles, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, and medical preparations containing active ingredients. Limited and high process complexity, to achieve the effect of photothermal ablation, strong photothermal conversion ability, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The preparation method of a drug-loaded nanoparticle of the present embodiment includes the following steps:
[0029] Step 1: Dissolve 0.0772 g of bismuth acetate (Bi(CHO)) in 30 mL of ethylene glycol to form a homogeneous solution, 0.175 g of UiO-66-NH sample is dissolved in 5 mL of ethylene glycol, and ultrasonically stir for 25-35 min. Stir slowly at room temperature in bismuth acetate for 55-65min;
[0030] Step 2: Add the NaS solution dropwise, continue to stir for 25-35min, then transfer the suspension to a stainless steel hydrothermal kettle, stand at 85-95°C for 0.8-1.2 hours, wash by centrifugation, ethanol and deionized water, And dried in an oven at 80 °C for 24 hours to obtain UiO-66 / BiS;
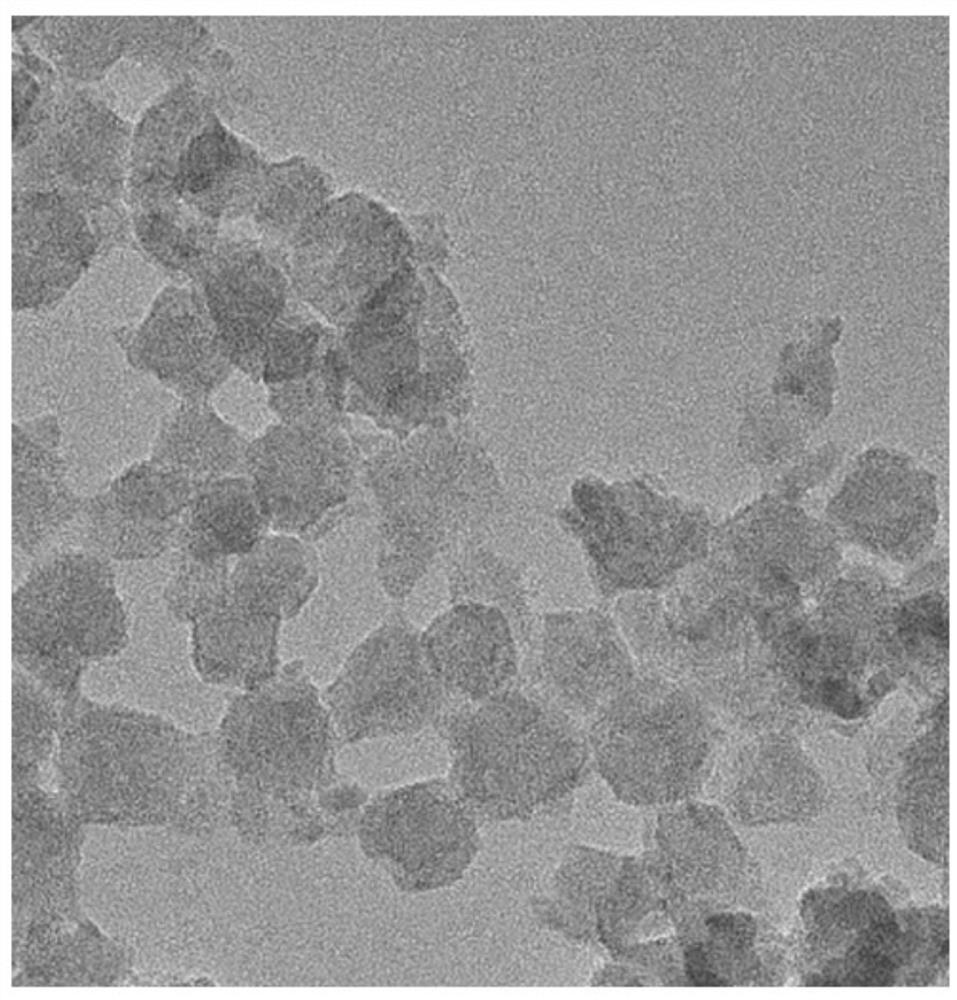
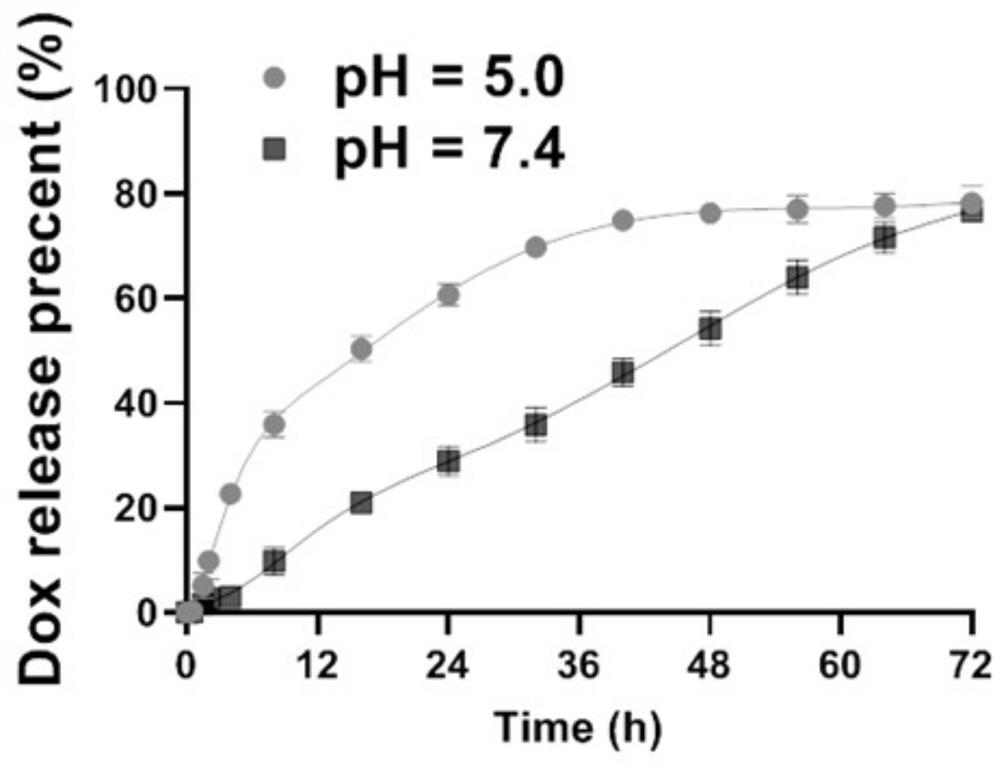
[0031] Step 3: Mix the prepared UiO-66 / BiS and DOX solution, stir overnight at room temperature in a dark environment, and obtain UiO-66 / BiS@DOX composite nanomaterials after centrifugation and washing with deionized water.
[0032] The addition amount of the NaS soluti...
Embodiment 1
[0039] In this embodiment,
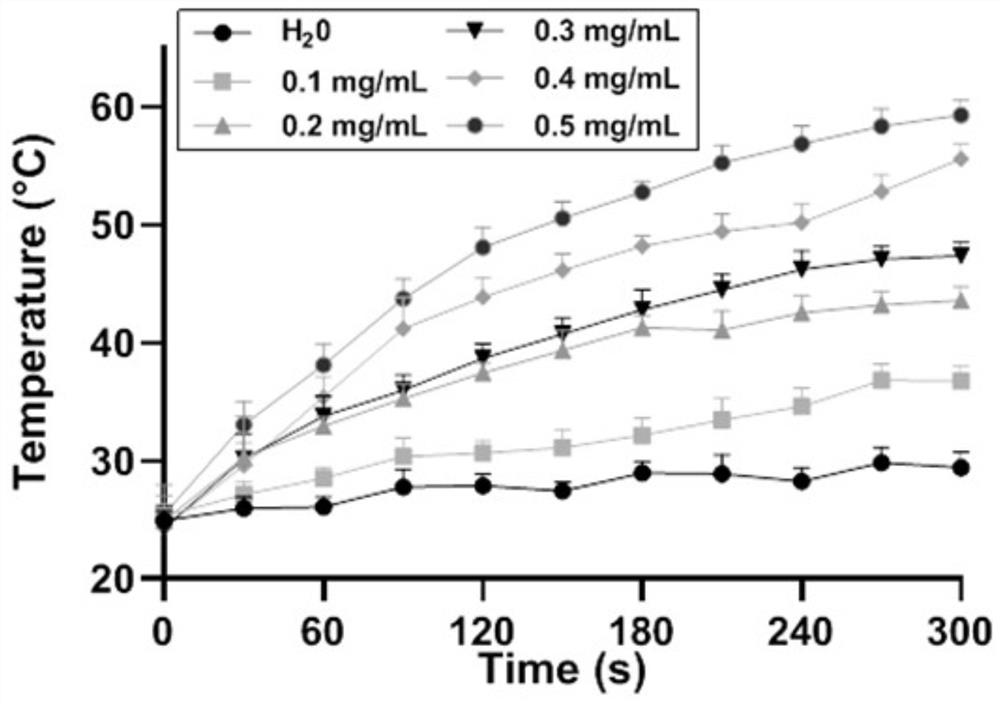
[0040] A drug-loaded nanoparticle for hepatic arterial chemoembolization in this embodiment is a novel doxorubicin-loaded metal-organic framework (MOF) nanoparticle; and a UiO-66 / Bi2S3 nanocomposite.
[0041] The preparation method of a drug-loaded nanoparticle of the present embodiment includes the following steps:
[0042] Step 1: Dissolve 0.0772 g of bismuth acetate (Bi(CHO)) in 30 mL of ethylene glycol to form a homogeneous solution, and dissolve 0.175 g of UiO-66-NH sample in 5 mL of ethylene glycol with ultrasonic stirring for 25 min, and then add it to bismuth acetate. Stir slowly at room temperature for 55min;
[0043] Step 2: Add the NaS solution dropwise, continue stirring for 25 min, then transfer the suspension to a stainless steel hydrothermal kettle, stand at 85°C for 0.8 hours, wash by centrifugation, ethanol and deionized water, and store in an oven at 80°C Dry for 24 hours to obtain UiO-66 / BiS;
[0044] Step 3: Mix the prepared ...
Embodiment 2
[0050] In this embodiment,
[0051]A drug-loaded nanoparticle for hepatic arterial chemoembolization in this embodiment is a novel doxorubicin-loaded metal-organic framework (MOF) nanoparticle; and a UiO-66 / Bi2S3 nanocomposite.
[0052] The preparation method of a drug-loaded nanoparticle of the present embodiment includes the following steps:
[0053] Step 1: Dissolve 0.0772 g of bismuth acetate (Bi(CHO)) in 30 mL of ethylene glycol to form a homogeneous solution, and dissolve 0.175 g of UiO-66-NH sample in 5 mL of ethylene glycol with ultrasonic stirring for 35 min, and then add it to bismuth acetate. Stir slowly at room temperature for 65 min;
[0054] Step 2: Add the NaS solution dropwise, continue stirring for 35 min, then transfer the suspension to a stainless steel hydrothermal kettle, stand at 95°C for 1.2 hours, wash by centrifugation, ethanol and deionized water, and store in an oven at 80°C Dry for 24 hours to obtain UiO-66 / BiS;
[0055] Step 3: Mix the prepared ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com