Preparation method of 2-bromo-N, N-dimethylaniline
A technology of dimethylaniline and o-bromoaniline, which is applied in the field of preparation of 2-bromo-N,N-dimethylaniline, can solve the problems of not reaching the scale-up production stage, low atom utilization rate, and increased cost expenditure. Achieve the effect of improving atom utilization rate, low cost and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
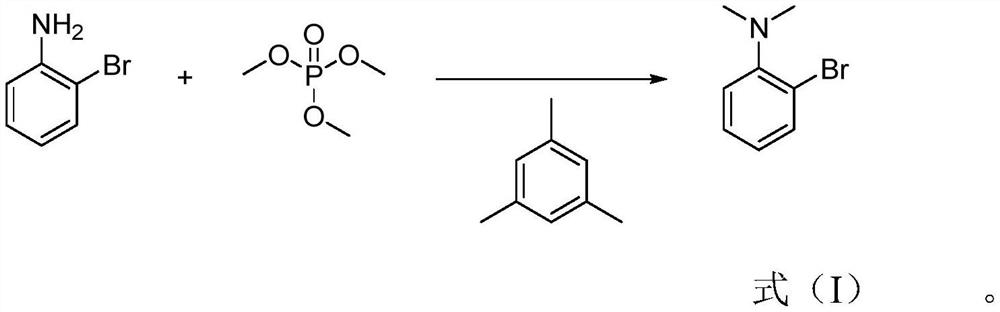
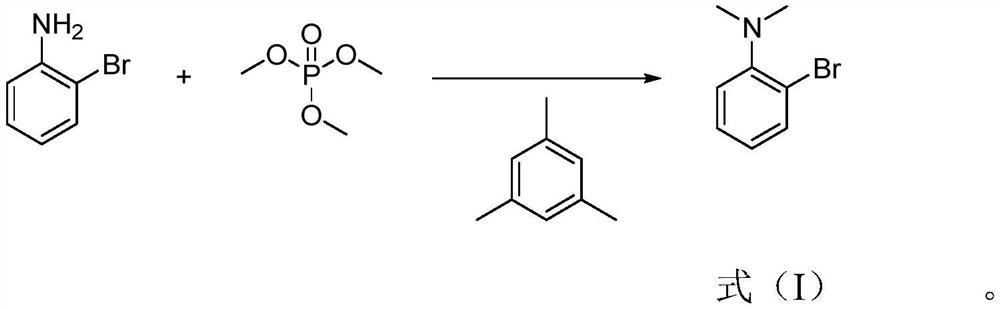
[0024] Specifically, the preparation method of 2-bromo-N,N-dimethylaniline of the present invention comprises: heating o-bromoaniline and mesitylene under stirring and controlling the temperature at 130-150° C., adding trimethyl phosphate dropwise , after the dripping is completed, after the incubation reaction for 3 to 6 hours, add an alkaline aqueous solution dropwise to the reaction solution to adjust the pH to be alkaline, extract with an extractant, separate the extractant, carry out desolvation of the extractant, and finally carry out rectification to obtain the formula 2-Bromo-N,N-dimethylaniline shown in (I).
[0025] The present invention uses mesitylene as the solvent of the methylation reaction, which can greatly reduce the violent exothermic phenomenon in the reaction process, effectively reduce the reaction temperature, and make the reaction milder. The reason may be that a large amount of The heat of reaction can be diluted by mesitylene, and the diluted heat is ...
Embodiment 1
[0038] 1kg of industrial pure o-bromoaniline (commercially available) and 1kg of mesitylene were added to the reaction flask, heated under stirring and temperature-controlled at 130~150 ℃, drip the trimethyl phosphate of 814g, after the dripping finishes. , under the condition of 140 ℃, stir for 4h, drop 20% NaOH aqueous solution to adjust the pH to be alkaline, extract with 0.5L dichloromethane, separate the dichloromethane phase, then carry out the precipitation of the dichloromethane phase, and finally carry out rectification to obtain The light yellow oily liquid was 569 g, the calculated yield was 49.1%, and the purity of 2-bromo-N,N-dimethylaniline obtained by gas chromatography was 98.0%. The molar ratio of trimethyl phosphate to o-bromoaniline in the present embodiment is 1, which is less than 1.5, and the yield is low.
Embodiment 2
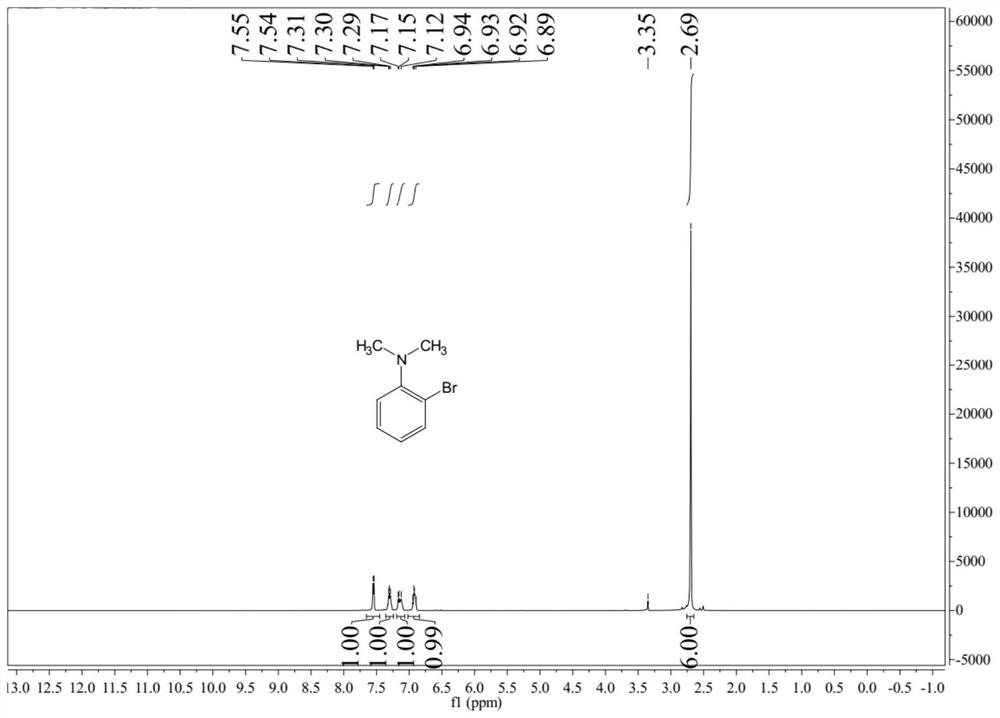
[0040]Add 1kg of industrial pure o-bromoaniline (commercially available) and 1kg of mesitylene into the reaction flask, heat under stirring and control the temperature at 130~150°C, add 1.63kg of trimethyl phosphate dropwise, and the reaction process is relaxed , after the dropwise addition, under the condition of 140 ℃, stir for 4h, drop 20% NaOH aqueous solution to adjust the pH to be alkaline, extract with 0.5L dichloromethane, separate the dichloromethane phase, and then carry out the precipitation of the dichloromethane phase, Finally, rectification was carried out to obtain 938 g of light yellow oily liquid, the calculated yield was 80.9%, and the purity of 2-bromo-N,N-dimethylaniline obtained by gas chromatography was 98.0%. figure 1 It is the nuclear magnetic spectrum of 2-bromo-N,N-dimethylaniline prepared in Example 2. The molar ratio of trimethyl phosphate and o-bromoaniline in the present embodiment is 2, and the yield is higher.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com