Method for preparing high-purity iron phosphate from pyrite cinder
A technology of pyrite slag and high-purity phosphoric acid, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of long process flow, large equipment investment, low utilization rate of pyrite slag, etc. To achieve the effect of shortening the process flow, improving the utilization rate, and reducing the cost of equipment investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
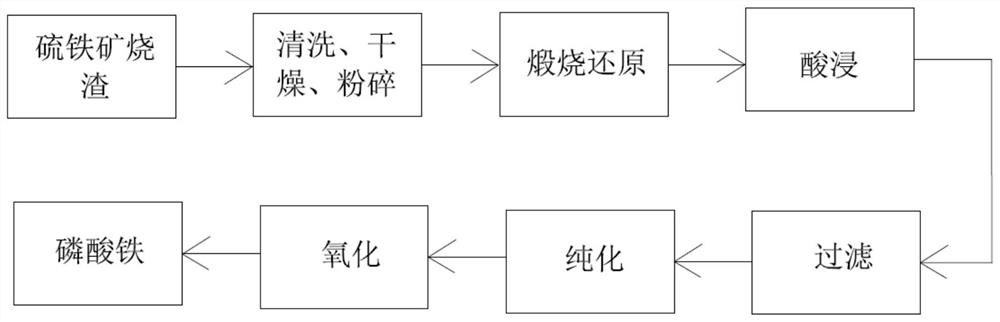
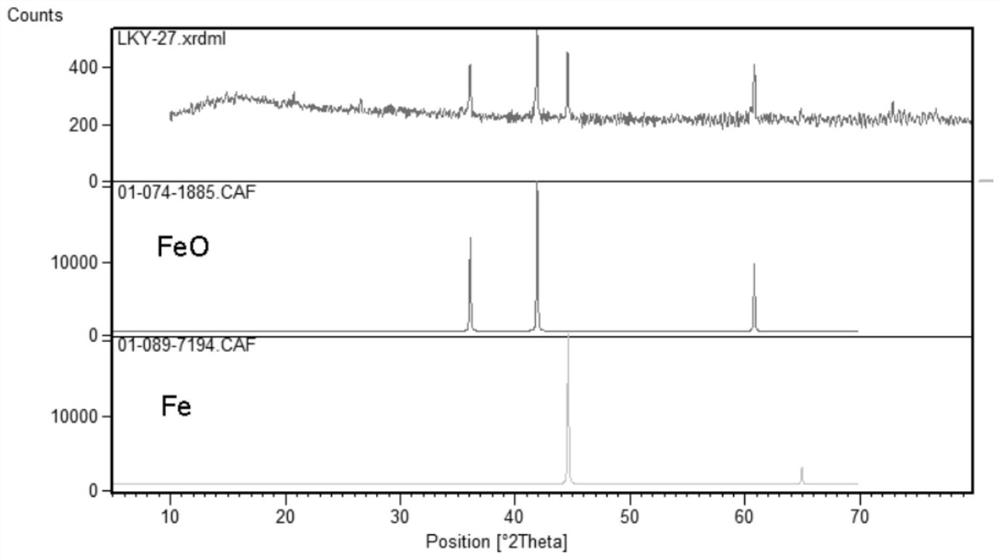
[0043] Wash the pyrite slag with deionized water according to the solid-to-liquid ratio of 20g / L, dry it at 120°C and grind it with a mortar for 30 minutes; weigh the glucose according to 50% of the mass of the obtained slag after grinding, put the pyrite slag into The slag was mixed with glucose, and calcined at 600°C for 4h in a tube furnace under nitrogen atmosphere; the iron content in the obtained sample after calcination was analyzed, and 20% dilute phosphoric acid was measured according to Fe:P=1:2 (molar ratio). , the calcined slag was mixed with dilute phosphoric acid; the mixed suspension was placed in a three-necked flask for 3 hours at 100 °C; after the reaction was completed, the resulting solution was subjected to suction filtration and washing; the filtrate obtained in the previous step was added to ammonia water to adjust pH=2.5 , and added polyacrylamide (mass concentration 0.02%), reacted for 2 hours and then filtered; analyze the iron content in the aforement...
Embodiment 2
[0045] The pyrite slag was washed with deionized water according to the solid-liquid ratio of 30g / L, dried at 120°C and ground with a mortar for 30 minutes; the glucose was weighed according to 20% of the mass of the obtained slag after grinding, and the pyrite slag was The slag was mixed with glucose, and calcined at 900°C for 2h in a tube furnace under nitrogen atmosphere; the iron content in the samples obtained after calcination was analyzed, and 40% dilute phosphoric acid was measured according to Fe:P=1:1.6 (molar ratio). , the calcined slag was mixed with dilute phosphoric acid; the mixed suspension was placed in a three-necked flask for 1 h at 150°C; after the reaction was completed, the resulting solution was subjected to suction filtration and washing; the filtrate obtained in the previous step was added to ammonia water to adjust pH=2.8 , and added polyacrylamide (mass concentration 0.02%), reacted for 2 hours and then filtered; analyze the iron content in the aforem...
Embodiment 3
[0047] The pyrite slag was washed with deionized water according to the solid-liquid ratio of 25g / L, dried at 120°C and ground with a mortar for 30 minutes; the glucose was weighed according to 10% of the mass of the obtained slag after grinding, and the pyrite slag was The slag was mixed with glucose, and calcined at 700°C for 6h in a tube furnace under nitrogen atmosphere; the iron content in the obtained sample after calcination was analyzed, and 60% dilute phosphoric acid was measured according to Fe:P=1:1.2 (molar ratio). , the calcined slag was mixed with dilute phosphoric acid; the mixed suspension was placed in a three-necked flask to react at 120 °C for 6 h; after the reaction was completed, the resulting solution was subjected to suction filtration and washing; the filtrate obtained in the previous step was added to ammonia water to adjust pH=4.0 , and added polyacrylamide (mass concentration 0.02%), reacted for 2 hours and then filtered; analyze the iron content in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com