Synthesis method of semaglutide
A synthesis method and technology of semaglutide, applied in the field of polypeptide drug production, can solve the problems of high separation and purification, limited coupling site, purification of polypeptide fragments, etc., and achieve the solution of purification difficulty, alleviation of difficulty and rapid transformation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
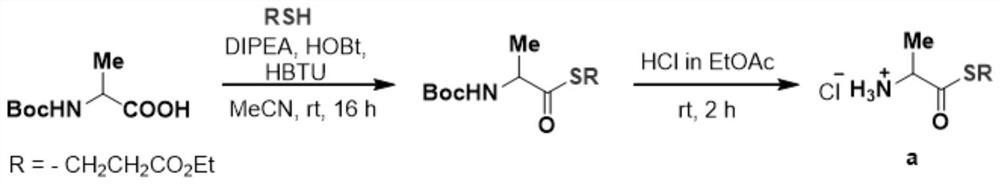
[0087] Example 1: Preparation of Intermediate a
[0088] Weigh Boc-Ala-OH (9.5 g, 50 mmol), HBTU (19.0 g, 55 mmol) and HOBT (8.1 g, 50 mmol) and dissolve in 250 mL of acetonitrile, add DIPEA (13.5 mL, 100 mmol), and activate at room temperature for 5 mins. Methyl thioglycolate (5.4 mL, 60 mmol) was added, and the mixture was reacted at room temperature for 16 hours. After the reaction, the acetonitrile was removed by rotary evaporation, an appropriate amount of ethyl acetate was added to dissolve the product, and 4% NaHCO was used for each. 3 , 1N HCl and saturated brine for three times, combine the organic phases, add anhydrous sodium sulfate, dry and filter, and remove the solvent. 150 mL of 2.0 mol / L hydrogen chloride ethyl acetate solution was added to react for 2 h. After the reaction, the solvent was removed by rotary evaporation, and then washed three times with ethyl acetate to obtain compound a, which was directly used in the next step without purification.
Embodiment 2
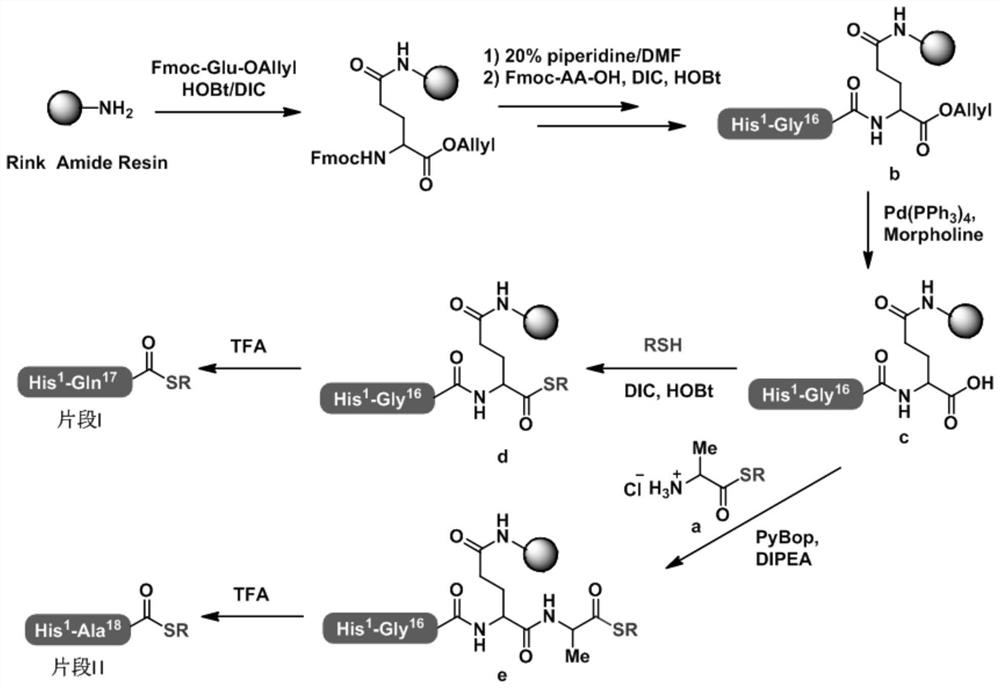
[0089] Example 2: Preparation of peptide c peptide resin
[0090] 46.6 g of Rink Amide resin with a substitution degree of 0.43 mmol / g was weighed into a solid-phase reaction column, and swollen with DMF / DCM (1:1) for 30 minutes. Deprotect with 200mLx2DBLK for 5min+7min, and wash with 200mLx5DMF. Weigh Fmoc-Glu-OAllyl (40.9 g, 100.0 mmol) and HOBT (16.2 g, 110.0 mmol) and dissolve them in 150 mL of DMF, add DIC (20.4 mL, 120.0 mmol) under ice bath for activation for 5 min, and add the mixture to the reaction In the column, the reaction was carried out at room temperature for 2 hours. At the end of the reaction, wash the resin with 200mLx3DMF, add 200mL DBLKx2 for deprotection 5min+7min, wash the resin with 200mLx5DMF, and continue to couple Fmoc-Gly-OH, Boc-Glu(OtBu)-OH, Fmoc-Leu-OH, Fmoc in sequence according to the peptide sequence -Tyr(tBu)-OH, Fmoc-Ser(tBu)-OH, Fmoc-Ser(tBu)-OH, Fmoc-Val-OH, Fmoc-Asp(OtBu)-OH, Fmoc-Ser(tBu)-OH, Fmoc-Thr(tBu)-OH, Fmoc-Phe-OH, Fmoc-Thr(tB...
Embodiment 3
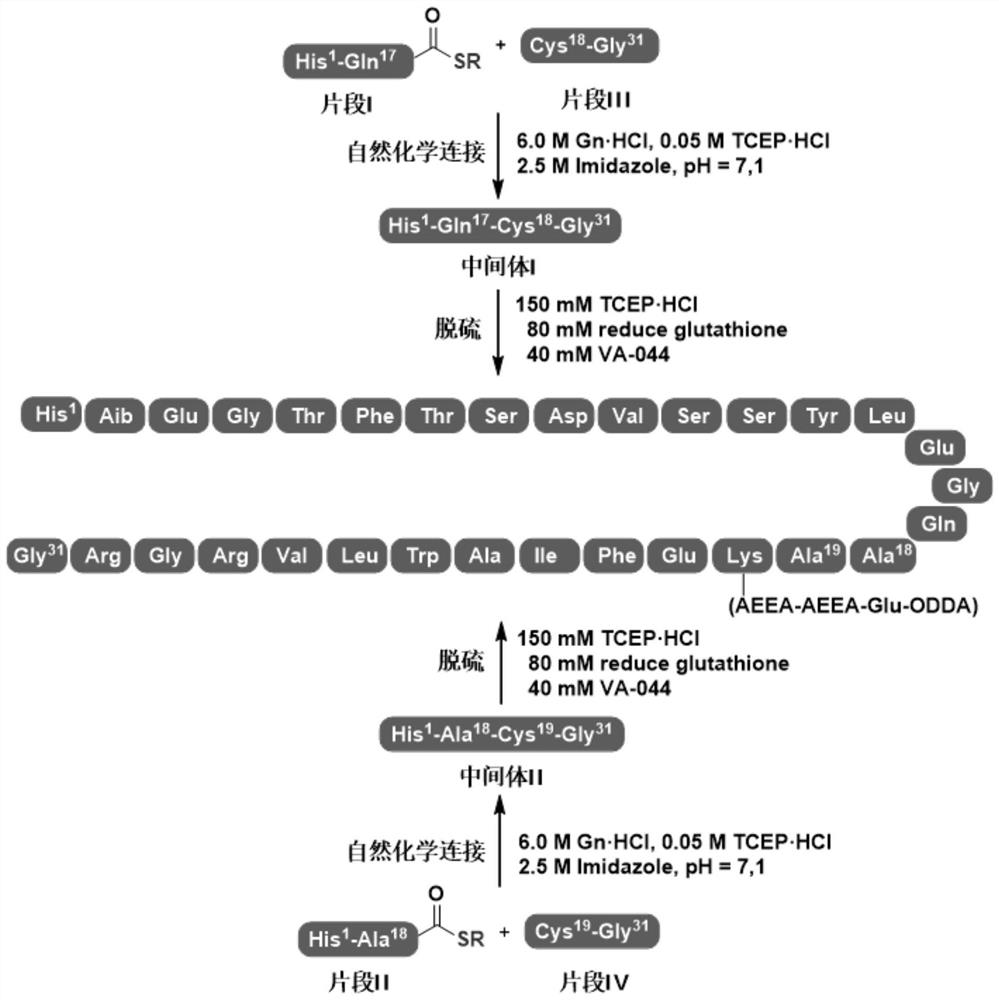
[0091] Example 3: Preparation of Peptide Fragment I
[0092] 44.3 g of the c-peptide resin obtained in Example 2 was weighed into a solid-phase reaction column and swollen with DMF for 30 minutes. Weigh HOBT (8.1 g, 55.0 mmol), DIC (10.2 ml, 60 mmol) and ethyl 3-mercaptopropionate (25.2 ml, 200 mmol), dissolve them in 150 mL of DMF, and add them to the reaction column. Repeat. After the reaction, the resin was washed with 150 mL x 5DMF, 150 mL x 3 MeOH was shrunk, and drained. Add the drained resin to a 1L round-bottomed flask, add the pre-configured lysis solution TFA / H 2 O / PhOMe / PhSMe (90:5:4:1, v / v) 500 mL, magnetically stirred at room temperature for 2 hours, filter the resin under reduced pressure, and collect the filtrate. The resin was washed with a small amount of TFA and the filtrates were combined. The filtrate was slowly added to 2.5L of ice ether for precipitation, centrifuged, washed with 2.5L×3 ice ether, and dried with nitrogen to obtain 21.5g of peptide fra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com