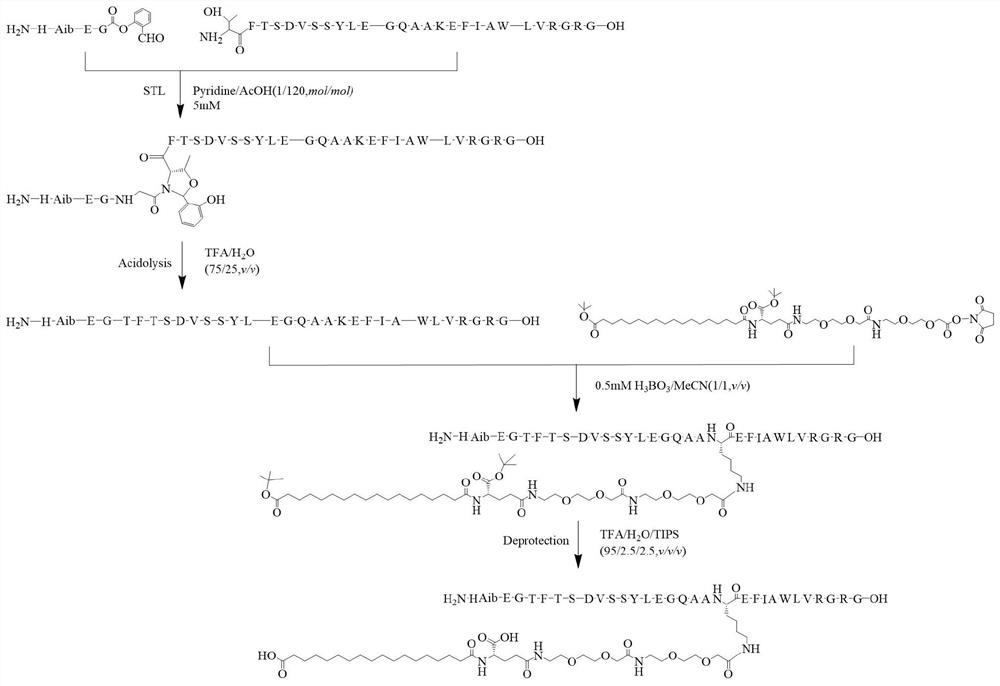
A kind of semi-synthetic preparation method of semaglutide
A semaglutide and compound technology, which is applied in the field of peptide synthesis, can solve the problems of strong steric hindrance effect, increased production cost, low product yield, etc., and achieves the effects of rapid transformation, easy operation and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1 Preparation of the crude product of compound I
[0075] Weigh out Boc-His(Trt)-Aib-Glu(Otbu)-Gly-OH (commercially purchased: purchased from Jill Biochemical (Shanghai), 1 g, 1.213 mmol), salicylaldehyde dimethyl acetal (407.8 mg, , 2.426mmol), PyBOP (946.8mg, 1.8195mmol), dissolve with 50mL DMF, add 600uLDIEA (3.639mmol) under the condition of ice-water bath, 0 ℃ of magnetic stirring for 20 hours. Complete conversion of the reaction was checked by liquid phase. After the reaction, the reaction solution was concentrated under reduced pressure, redissolved by adding DCM, washed with saturated brine three times, the collected DCM solution was put into a certain amount of anhydrous sodium sulfate for drying, left standing for 0.5 hours, suction filtered, and the filtrate was collected. It was drained to obtain 1.89 g of compound 0 crude product, which was used.
[0076]
[0077] Take all the samples of above compound 0, add 50mL cleavage reagent (TFA / H 2 0 / T...
Embodiment 2
[0079] Example 2 Purification of the crude product of compound I
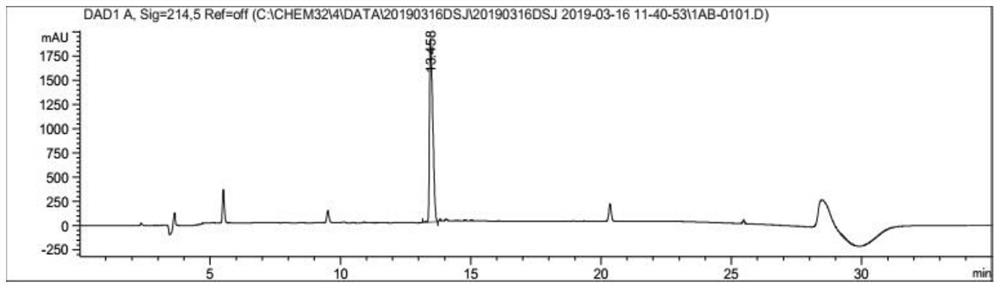
[0080] Weigh 500 mg of the crude product of Compound I prepared in Example 1, add 15 mL of distilled water to dissolve, use Water1525 system for semi-preparative purification, the wavelength is 214 nm, and the chromatographic column is 20 × 250 mm reverse-phase C 18 Column, the column temperature is 37°C, the mobile phase is water (phase A) containing 0.1% TFA and acetonitrile (phase B) containing 0.1% TFA, the flow rate is 8ml / min, gradient: B%: 20%-60%, After 30 min, the target fraction was collected, and the collected solution was concentrated under reduced pressure.
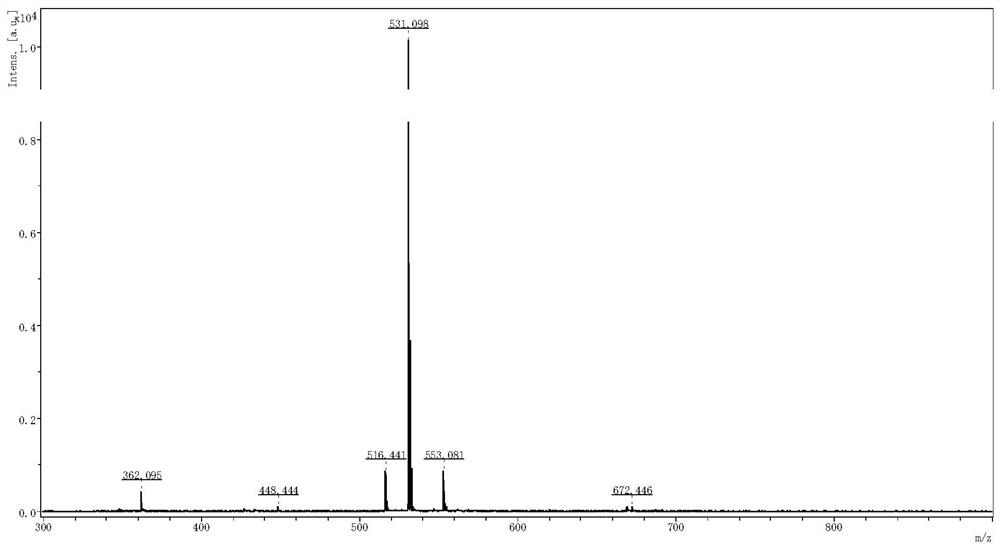
[0081] MAIDI-TOFMS was used for detection, and the results are shown in figure 2 , MAIDI-TOFMS calculates C 24 H 30 N 6 O 8 Theoretical molecular weight [M+H] + m / z=531.220,[M+Na] + = 553.202, observed: 531.098, 553.081. The above concentrated solution was lyophilized to obtain 195 mg of compound I in the form of a white solid powder ...
Embodiment 3
[0083] Example 3 Synthesis of Compound II
[0084] Weigh compound I (50mg, 0.094mmol), Arg34GLP-1 (11-37) (150mg, 0.05mmol) respectively, dissolve with 100ul pyridine acetate buffer solution (acetic acid:pyridine, mol:mol, 120:1), 35 The reaction was magnetically stirred for 20 hours. The solvent was removed by blow-drying, then the residue was acidolyzed with 10 mL of acid hydrolysis solution (TFA / water, 75 / 25, v / v) and magnetically stirred at room temperature for 2 hours. The solvent was removed by blow-drying, and lyophilized to obtain 230 mg of the reaction mixture. Analytical liquid phase analysis was carried out on the reaction mixture (using liquid phase conditions: using Agilent 1260 system for liquid phase analysis, wavelength 214nm, the chromatographic column was a reversed-phase C18 column of 4.6×250mm, the column temperature was 35°C, and the mobile phase was 0.1% of water (phase A) and 0.1% acetonitrile (phase B), the flow rate is 1 mL / min, gradient: B%: 30%-45%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com