Vaccine delivery vector as well as preparation method and application thereof
A delivery carrier and vaccine technology, applied in the field of vaccine delivery carrier and its preparation, can solve problems such as design difficulties and limited materials, and achieve the effects of improving delivery efficiency, reducing difficulty, and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Synthesis of PAMAM-R848 polymer
[0058] The immunoadjuvant-bonded polymer is obtained by Michael addition reaction between the amino group on the surface of the dendritic PAMAM polymer and the double bond of the R848 disulfide derivative.
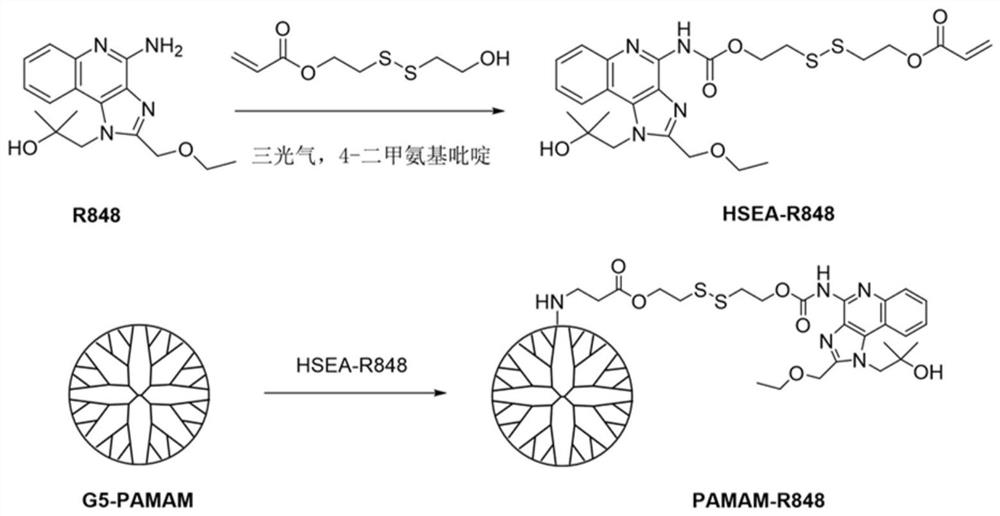
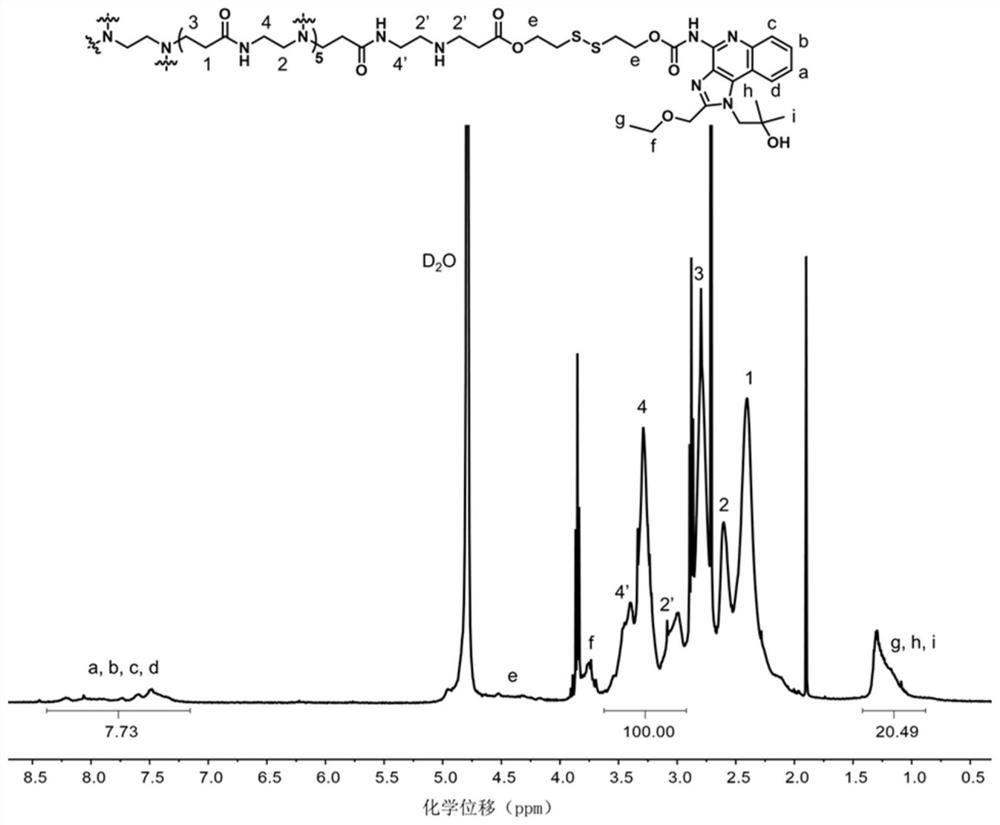
[0059] In this embodiment, the dendritic PAMAM polymer is the fifth-generation PAMAM polymer, the molecular weight of the fifth-generation PAMAM polymer is 28826 g / mol, and 1 mol of the fifth-generation PAMAM contains 128 mol of amino groups. The synthetic route of PAMAM-R848 is as follows figure 1 shown.
[0060] The specific preparation steps of the immunoadjuvant-bonded polymer (ie PAMAM-R848) are as follows: 15 mg of fifth-generation PAMAM (G5-PAMAM) and 17.1 mg of HSEA-R848 are dissolved in 1.0 mL of anhydrous DMSO, HSEA-R848 and G5 - The molar ratio of PAMAM was 60:1, and the reaction was carried out in an oil bath at 40°C for 48 hours; the product was passed through a PD-10 gel column with ultrapure water as the ...
Embodiment 2
[0062] Example 2: PAMAM-R848 polymer and antigenic protein to prepare nanovaccine
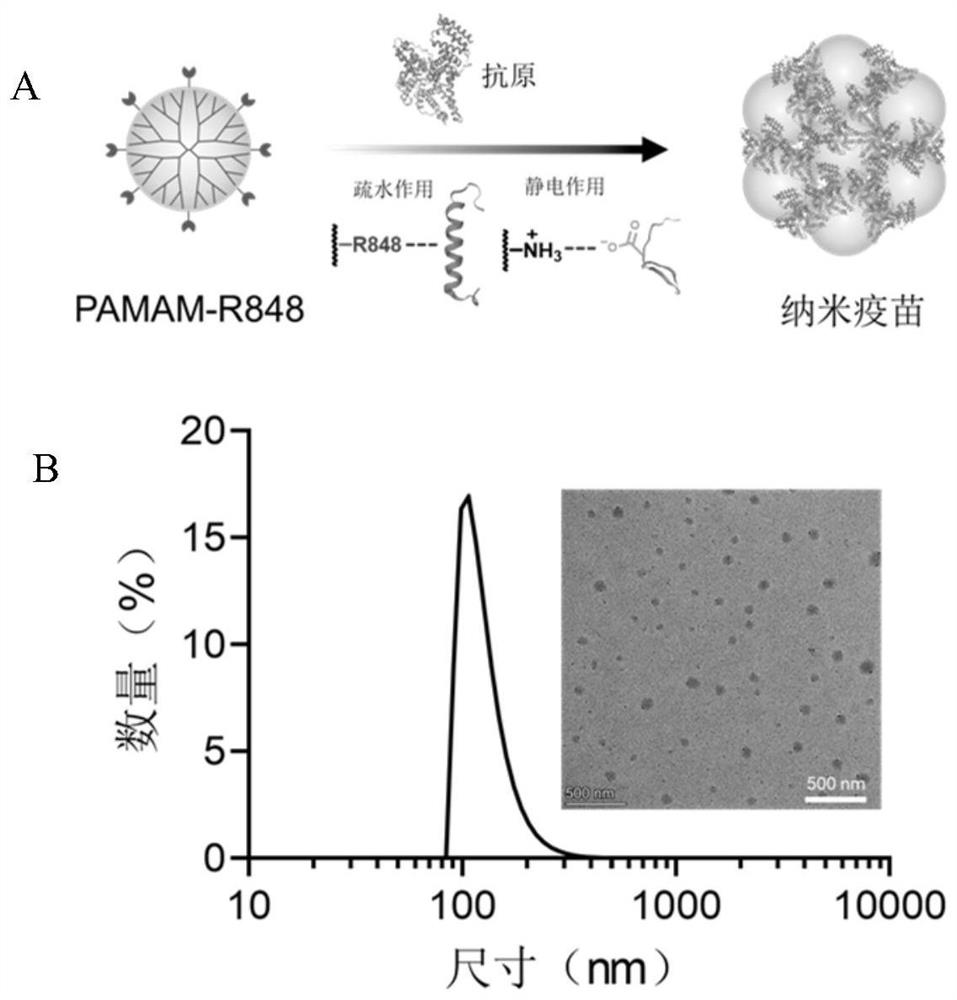
[0063] The schematic diagram of the formation of nanovaccine between PAMAM-R848 polymer and protein antigen through electrostatic interaction and hydrophobic interaction is shown in Fig. image 3 shown in A.
[0064] In the verification experiment, a PAMAM-R848 aqueous solution with a concentration of 0.1-1 mg / mL was prepared, and then OVA model antigen was added at mass ratios of 2:1, 1:1, and 1:2 (polymer: antigenic protein), and vortexed. Mix well (the rotation speed is 500-2500rpm, just mix well) and incubate for 10 minutes to obtain the PAMAM-R848 / OVA (referred to as PRO) nano-vaccine. The PRO nanovaccine prepared with a mass ratio of 2:1 is denoted as PRO(2:1), and its dynamic light scattering (DLS) and transmission electron microscopy (TEM) characterization results are as follows image 3 As shown in B, the results show that the PRO(2:1) particle size is about 100 nm, and the morpholog...
Embodiment 3
[0066] Example 3: In vitro immune activation and antigen delivery effect of nanovaccine
[0067] As a TLR 7 / 8 agonist, R848 can promote the maturation of DC cells. In order to identify the immune activation function of PRO at the cellular level, the present invention selects the nanovaccine with three mass ratios obtained by the preparation method of Example 2: PRO (1:2 ), PRO(1:1), PRO(2:1). In the present invention, these three nano-vaccine and successfully induced BMDCs are incubated together for 24 hours, and the concentration of OVA is 10 μg / mL, and then CD11c, CD80 and CD86 antibodies are used to label the surface molecules of BMDCs, and flow cytometry is used to detect the expression level of costimulatory signal molecules , the streaming statistics are as follows Figure 4 shown in A. Compared with the control group (without vaccine and OVA) and the free OVA group (without immune adjuvant), the PRO vaccine can effectively promote the maturation of BMDCs in vitro, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com