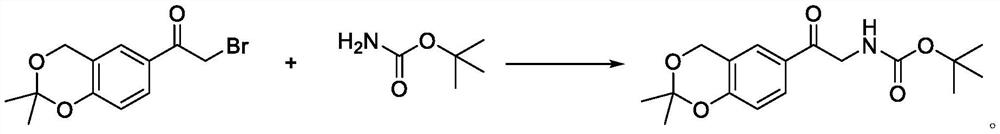
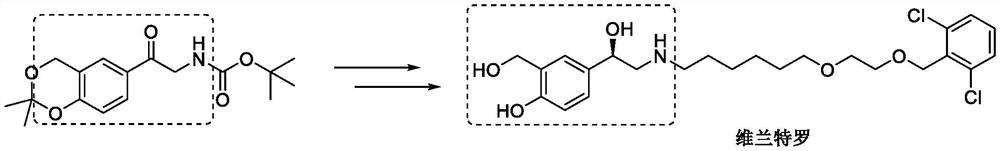
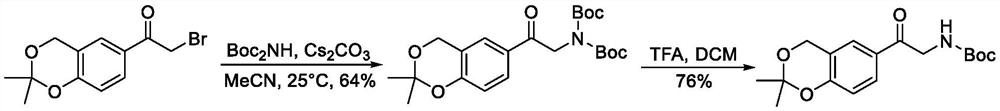
Method for synthesizing vilanterol intermediate from tert-butyl carbamate
A technology of carbamic acid and tert-butyl ester, applied in organic chemistry, bulk chemical production, etc., can solve the problems of low total yield, long reaction steps, poor selectivity of Boc protecting group removal by trifluoroacetic acid, etc., and reduce production cost, avoiding poor selectivity, and shortening the effect of synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] At room temperature, add tert-butyl carbamate (225g, 1.1eq) to a 5L three-necked flask, add 500mL of tetrahydrofuran, stir to dissolve, bring the system to -5-0°C, and slowly dropwise add LDA (960mL, 2mol / L, 1.1 eq) solution, keep stirring for 0.5h after adding, then slowly add 6-bromoacetyl-2,2-dimethyl-4H-benzo[1,3]dioxin (500g, 1.0eq) and 1L The solution prepared with tetrahydrofuran was added dropwise to the reaction system, and the reaction was incubated for 2h after the addition. The middle control reaction was completed, the reaction solution was concentrated, then saturated sodium bicarbonate solution (2L) and ethyl acetate (2L) were added, the residue was stirred and dispersed, and the layers were separated. The organic layer was water (2L) and saturated brine (2L) respectively. Washed, then dried with anhydrous sodium sulfate, filtered to remove magnesium sulfate, concentrated in vacuo to remove solvent, added isopropyl ether (1 L), stirred for crystallization...
Embodiment 2
[0021] To a 5L three-necked flask at room temperature, add tert-butyl carbamate (225g, 1.1eq), add tetrahydrofuran (500mL), stir to dissolve, bring the system to -5-0°C, slowly add potassium tert-butoxide (217g, 1.1eq) ) solution, keep stirring for 0.5h after adding, then slowly mix 6-bromoacetyl-2,2-dimethyl-4H-benzo[1,3]dioxin (500g, 1.0eq) and 1L tetrahydrofuran The prepared solution was added dropwise to the reaction system, and the reaction was incubated for 2h after the addition. The middle control reaction was completed, the reaction solution was concentrated, then saturated sodium bicarbonate solution (2L) and ethyl acetate (2L) were added, the residue was stirred and dispersed, and the layers were separated. The organic layer was water (2L) and saturated brine (2L) respectively. Washed, then dried with anhydrous sodium sulfate, filtered to remove magnesium sulfate, concentrated in vacuo to remove solvent, added isopropyl ether (1 L), stirred for crystallization at roo...
Embodiment 3
[0023] Add tert-butyl carbamate (225g, 1.1eq) to a 5L three-necked flask at room temperature, add tetrahydrofuran (500mL) and stir to dissolve. At room temperature, slowly add potassium tert-butoxide (217g, 1.1eq) solution, and stir after adding 0.5h, then slowly add the solution prepared by 6-bromoacetyl-2,2-dimethyl-4H-benzo[1,3]dioxin (500g, 1.0eq) and 1L tetrahydrofuran into the reaction system dropwise , and the reaction was incubated for 2h after the addition. The middle control reaction was completed, the reaction solution was concentrated, then saturated sodium bicarbonate solution (2L) and ethyl acetate (2L) were added, the residue was stirred and dispersed, and the layers were separated. The organic layer was water (2L) and saturated brine (2L) respectively. Washed, dried with anhydrous sodium sulfate, filtered to remove magnesium sulfate, concentrated in vacuo to remove solvent, added isopropyl ether (1 L), stirred for crystallization at room temperature, filtered t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com