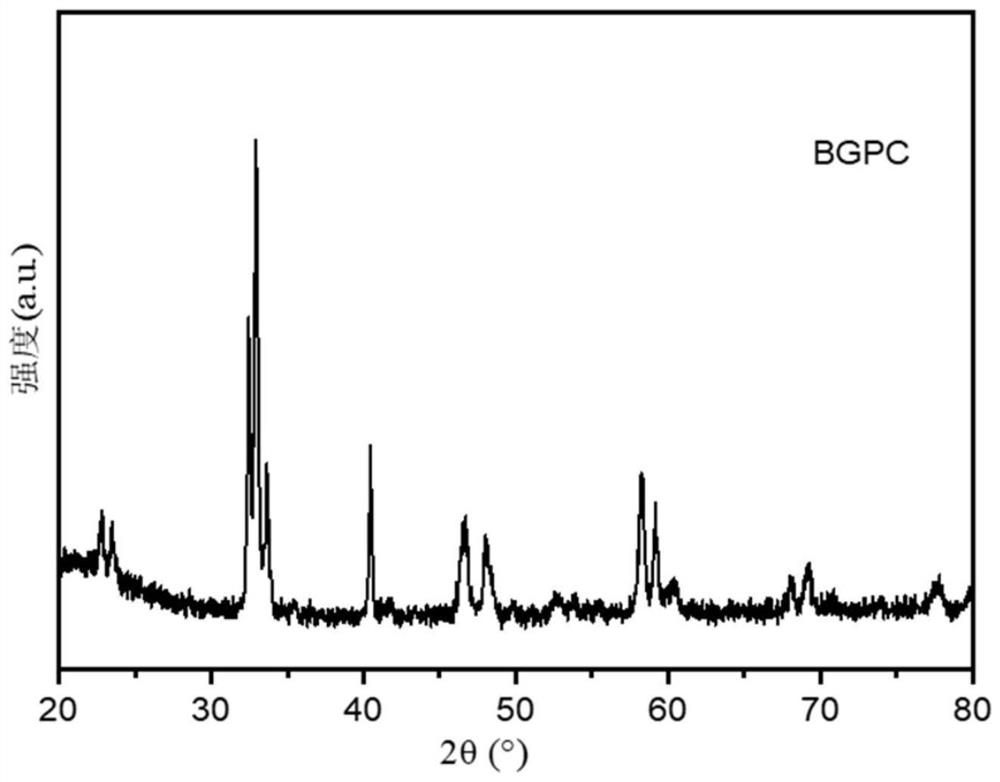
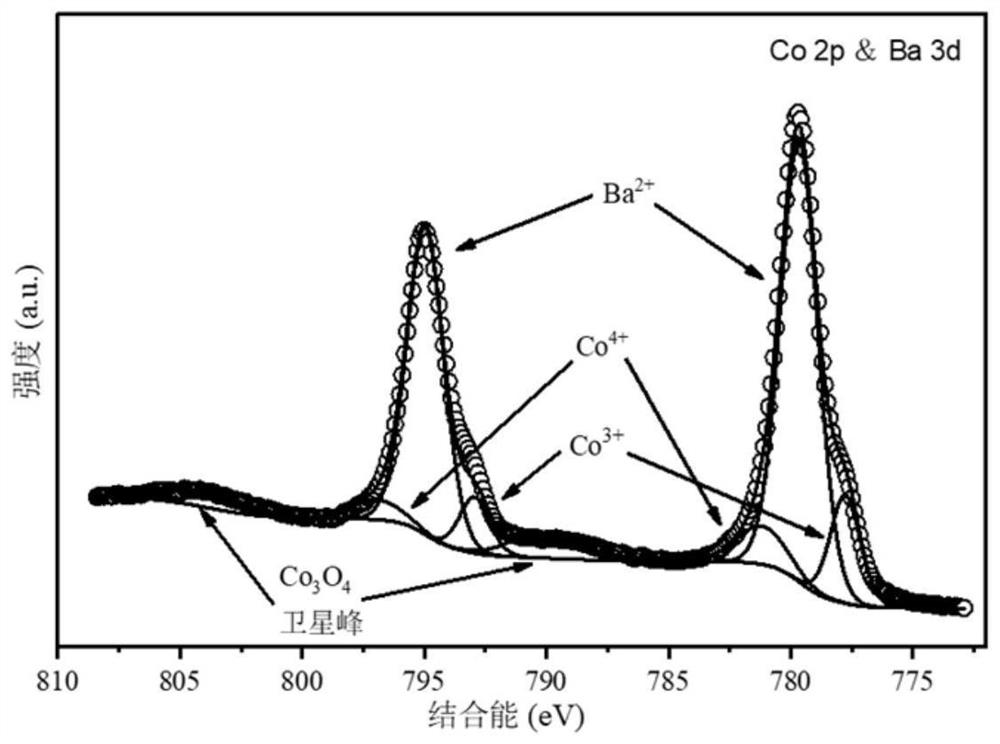
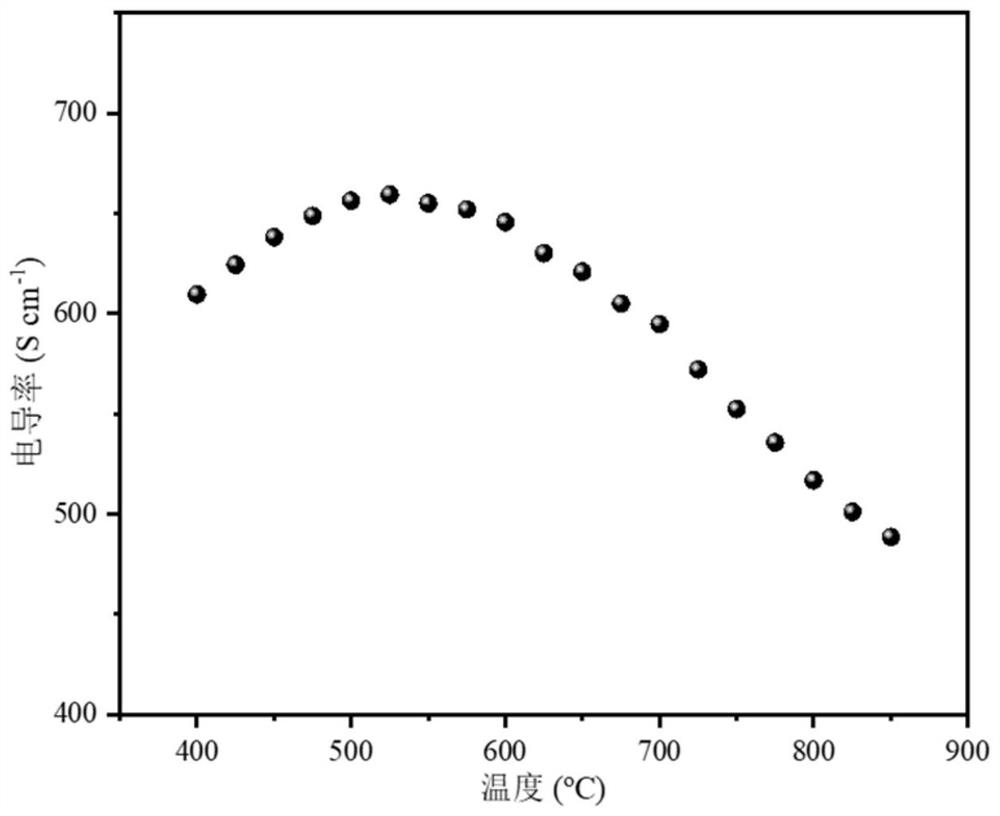
Stable solid oxide fuel cell cathode material realized by A-site regulation and preparation and application thereof
A fuel cell cathode, solid oxide technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of decreased battery performance, increased battery area, slow kinetic process, etc., to achieve low activation energy, high power density, The effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] This embodiment provides a stable solid oxide fuel cell cathode material Ba realized by A-site regulation 0.8 Gd 0.8 Pr 0.4 Co 2 O 6-δ The preparation method specifically comprises the following steps:
[0038] 1) Take by weighing the barium nitrate of 4.182g, the gadolinium nitrate of 7.222g, the praseodymium nitrate of 3.480g, the cobalt nitrate of 11.641g, add deionized water to dissolve completely; Press metal ion (Ba, Gd, Pr and Co): Glycine: The molar ratio of citric acid monohydrate is 4:3:3. Weigh 4.504g of glycine and 12.608g of citric acid monohydrate as complexing agents, and add them to the above solution to obtain a mixed solution;
[0039] 2) The mixed solution is heated and stirred at a constant temperature of 120 ° C until the water evaporates to dryness to form a gel-like substance;
[0040] 3) heating the gelatinous substance in an oven at 300° C. to complete combustion, and keeping the temperature for 5 hours to obtain fluffy cathode material pre...
Embodiment 2
[0043] This embodiment provides a Ba 0.8 Gd 0.8 Pr 0.4 Co 2 O 6-δ A symmetrical battery is prepared for the battery cathode. The symmetrical battery adopts a "cathode|electrolyte|cathode" structure, which specifically includes the following steps:
[0044] 1) Weigh 1 g of the cathode powder obtained in Example 1, and weigh 0.76 g of terpineol and 0.04 g of ethyl cellulose according to the powder: terpineol: ethyl cellulose mass ratio of 100:76:4 Place in a mortar and grind to make cathode slurry;
[0045] 2) Evenly brush the cathode slurry on the dense LSGM (La 0.8 Sr 0.2 Ga 0.8 Mg 0.2 O 3-δ ) on both sides of the electrolyte sheet, sintered at 1050 °C for 2 h in an air atmosphere to form a layered double perovskite Ba 0.8 Gd 0.8 Pr 0.4 Co 2 O 6-δ Porous electrode, the effective cathode area of the battery is 0.47cm 2 , to obtain a symmetrical battery; it is used for the polarization impedance test of the cathode material in the temperature range of 600-800 °C...
Embodiment 3
[0047] This embodiment provides a Ba 0.8 Gd 0.8 Pr 0.4 Co 2 O 6-δ A single cell is prepared for the battery cathode. The single cell adopts the "Ni-GDC|LDC|LSGM|Cathode" structure, which specifically includes the following steps:
[0048] 1) Weigh 1 g of the cathode powder obtained in Example 1, and weigh 0.76 g of terpineol and 0.04 g of ethyl cellulose according to the powder: terpineol: ethyl cellulose mass ratio of 100:76:4 Place in a mortar and grind to make cathode slurry;
[0049] 2) The cathode slurry was evenly brushed on the surface of the LSGM electrolyte, and sintered at 1050 °C for 2 h in an air atmosphere to form a layered double perovskite Ba 0.8 Gd 0.8 Pr 0.4 Co 2 O 6-δ Porous electrode, the effective cathode area of the battery is 0.2826cm 2 ; A transition layer LDC is prepared on the other side of the electrolyte, and an anode Ni-GDC is prepared on the other side of the transition layer; it is used for the output power test of the cathode material...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com