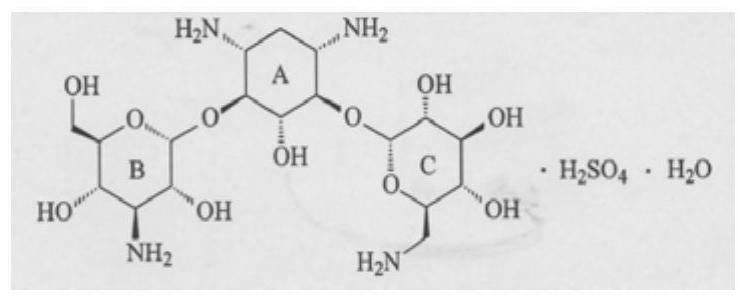
Method for preparing kanamycin monosulfate single crystal and application thereof
A technology of kanamycin monosulfate and single crystal, which is applied in the field of medicine and can solve the problems of inconvenient storage and transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Example 1. Preparation of single crystal of kanamycin monosulfate
[0093] The present embodiment prepares the single crystal of kanamycin monosulfate, and the operation steps are as follows:
[0094] (1) Take 1000 mg of kanamycin monosulfate bulk drug, put it in a 50 ml Nessler colorimetric tube, add 20 ml of decarbonated water to dissolve, measure the pH value and adjust the pH value of the solution to 6.0~6.2 with 1M sulfuric acid solution , then add 1ml of isopropanol to mix;
[0095] (2) 10ml methanol is slowly added from the liquid level of the solution obtained in step (1) at a speed of 2ml / min under the temperature condition of 22~25°C;
[0096] (3) stand for 24 hours under the above-mentioned temperature conditions, precipitate columnar crystals, and take out. The obtained crystal may be referred to as kanamycin A monosulfate in the present invention.
[0097] The decarbonated water used was water obtained by boiling double-distilled water for 10 minutes a...
Embodiment 2
[0098] Example 2. Determination of kanamycin monosulfate crystals by single crystal X-ray diffraction (XRD)
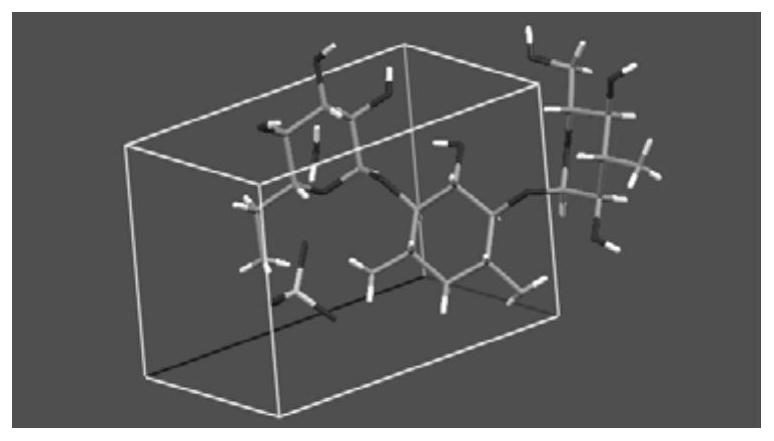
[0099] In this example, a single crystal X-ray diffractometer (XRD) was used to measure the columnar crystals of kanamycin monosulfate obtained in Example 1 to prove its crystal structure.
[0100] According to the analysis of the collection of kanamycin monosulfate in the pharmacopoeia of various countries, including ChP2000 version, USP39, EP8.0, BP2016 and JP16, the difference of water molecules in the molecular formula of kanamycin monosulfate is recorded in this example. The kanamycin monosulfate crystals obtained by culture were measured by a single crystal X-ray diffractometer, the molecular formula of the obtained kanamycin monosulfate crystals was verified, and the absolute configuration, Conformation, unit cell, hydrogen bond and salt bond arrangement within unit cell.
[0101] Sample: the columnar crystal of kanamycin monosulfate obtained in Example 1, th...
Embodiment 3
[0132] Example 3. Consistency proof of kanamycin monosulfate single crystal and kanamycin monosulfate raw material crystal
[0133] In this example, the consistency of the crystals of the kanamycin monosulfate crystal obtained in Example 1 (kanamycin monosulfate A, single crystal) and the used kanamycin monosulfate raw material drug is verified.
[0134] 1. Electron microscope
[0135] Preliminary inspection was carried out by optical polarizing microscope, which proved that the commercial kanamycin monosulfate bulk drug had crystallinity. After recrystallization in Example 1, a new crystal (kanamycin monosulfate A) was obtained. The properties of the crystals were observed under a cold field emission scanning electron microscope. Figure 5 The electron microscope crystal structure of kanamycin monosulfate.
[0136] 2. Powder X-ray Diffraction
[0137] In order to further confirm the crystallinity of kanamycin monosulfate, the powder X-ray diffraction patterns of the raw ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com