Application of neferine in preparation of anti-hepatitis B virus drug
A technology of hepatitis B virus and liensinine, which is applied in the field of medicine and biology, to achieve remarkable drug effects, easy promotion, and easy results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
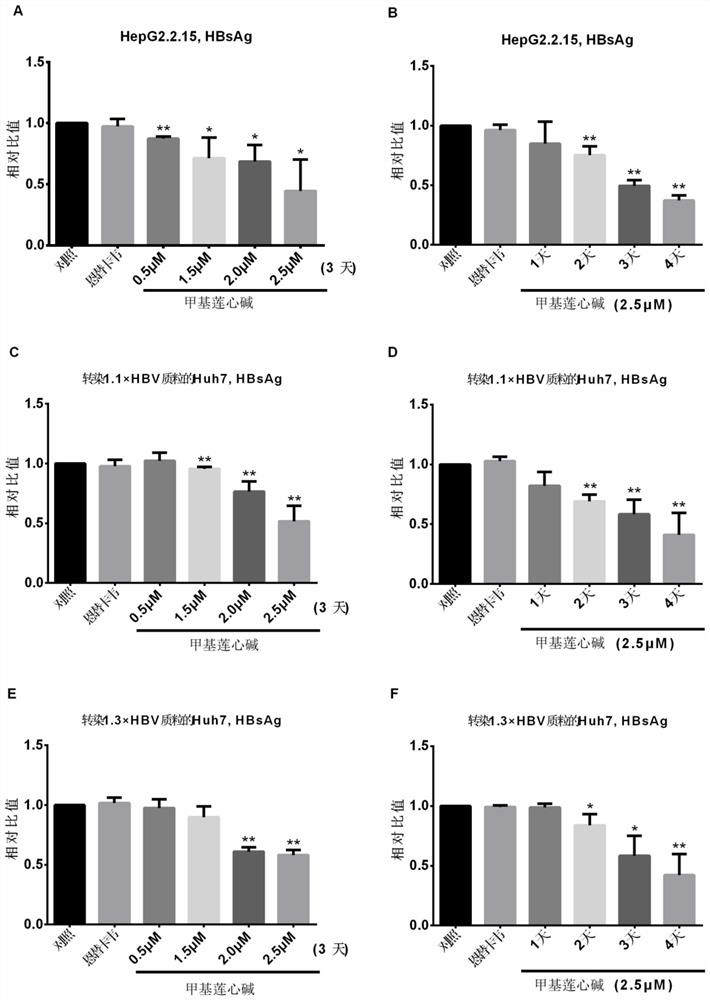
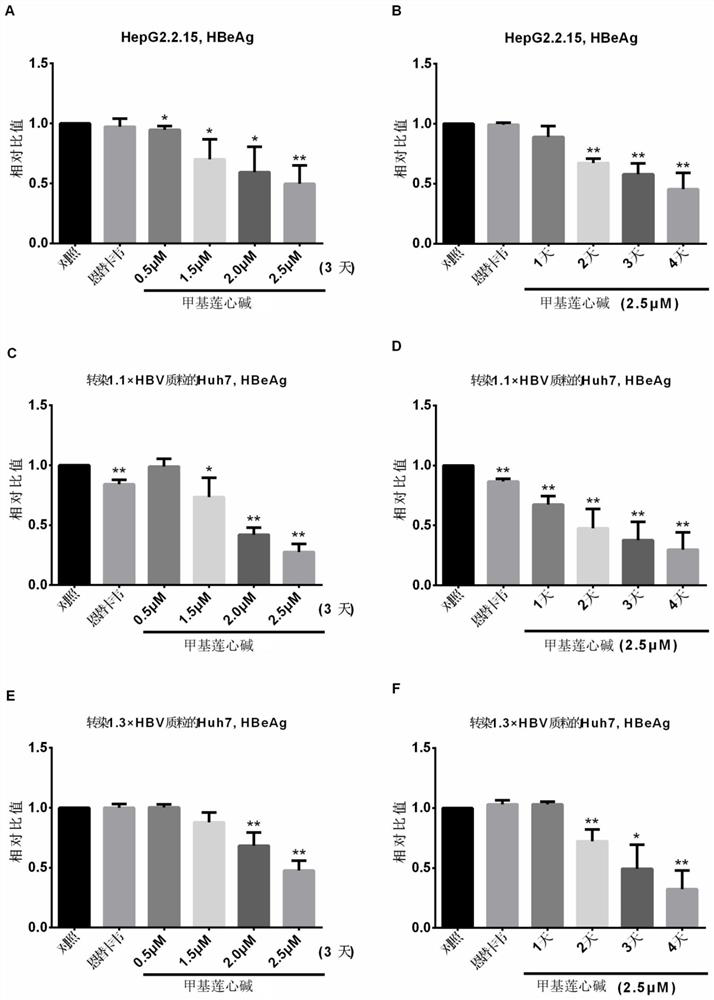
[0024] The changes of HBsAg and HBeAg levels in the supernatant of the cells treated with lysinine were detected by enzyme-linked immunosorbent assay (ELISA)
[0025] Step 1. Inoculate liver cancer cells in six-well plates, and start adding drug stimulation 1 day after HepG2.2.15 inoculation, or 1 day after Huh7 transfection with 1.1 / 1.3 x HBV plasmid. Add 0.5 μM, 1.5 μM, 2.0 μM and 2.5 μM to the medium in turn, and add 30 nM ETV to the medium of the positive control. The treatment time is 3 days, and the medium is changed every day. 20°C for use.
[0026] Among them, the 1.1 x HBV plasmid used is pCH9-3091, which contains 1.1 times the HBV genome of the adw genotype, and its upstream is promoted by a human cytomegalovirus (HCMV) type 1 early-mid protein (IE1) promoter, which is a strong promoter After the plasmid is transfected into mammalian eukaryotic cells, the HBV RNA transcript can be transcribed with high efficiency. This plasmid was a gift from Dr. Michael Nassal, Un...
Embodiment approach 2
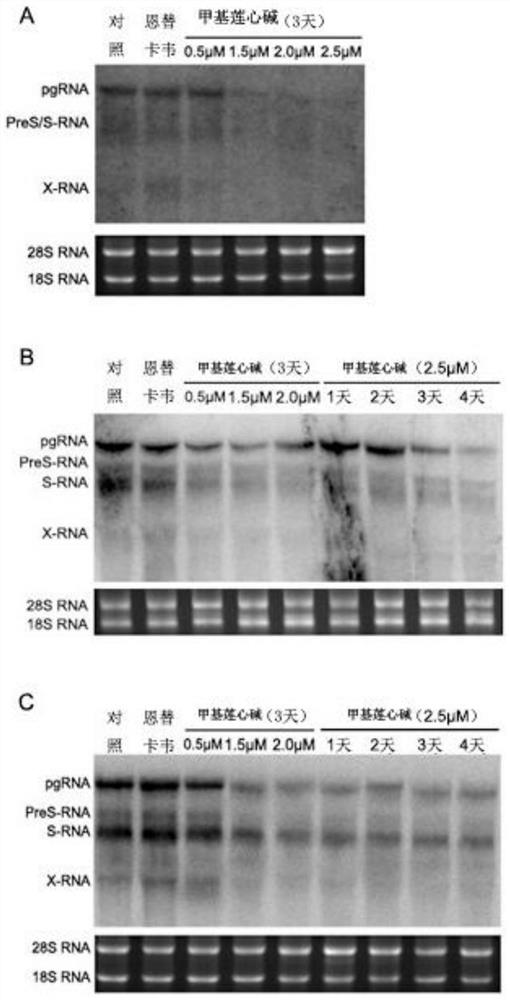
[0040] Detection of intracellular viral mRNA levels by Northern blotting
[0041] Step 1. Wash the cells of each group used to detect HBsAg and HBeAg in the supernatant three times with PBS, then add 1 mL of Trizol solution to each well of the six-well plate, digest at room temperature for 5 minutes, and shake Trizol together with the cells. Transfer to a new centrifuge tube and pipette repeatedly until no obvious precipitation occurs.
[0042] Step 2. Add 1 / 5 volume of chloroform, shake vigorously until the solution becomes milky white, and let stand for 5 minutes at room temperature.
[0043] Step 3. Centrifuge at 12000g for 15min at 4°C. After centrifugation, the mixture is divided into 3 layers: the colorless supernatant containing RNA, the genomic DNA in the middle, and the colored lower organic phase. Transfer the supernatant carefully to another. in a new centrifuge tube.
[0044] Step 4. Add an equal volume of isopropanol, mix well, and let stand at room temperature ...
Embodiment approach 3
[0062] Detection of viral DNA levels in intracellular HBV nucleocapsids by Southern blotting
[0063] Step 1. Inoculate HepG2.2.15 in a 10cm petri dish, and inoculate Huh7 in a 6cm petri dish. After 1 day of HepG2.2.15 inoculation, or 1 day after Huh7 was transfected with 1.1 / 1.3x HBV plasmid, drug stimulation was started. Add 0.5 μM, 1.5 μM, 2.0 μM and 2.5 μM lysinine, and 30 nM ETV as a positive control to the culture medium, the treatment time is 3 days, the medium is changed every day, and the cells are cryopreserved at -80 °C after 3 days. use;
[0064] Step 2. Add 2.5 μM lysinine and 30 nM ETV as a positive control to the culture medium of the cells with the same conditions in Step 1 for 4 days, change the medium every day and collect the cells from the first day to the fourth day of treatment. Frozen at -80℃ for later use;
[0065] Step 3. Add cell lysate to each sample, add 1mL of lysate to cells in a 10cm dish, add 500μL of lysate to a 60mm dish, shake for 30min at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com