Chromium-cerium-zirconium-aluminum solid solution material as well as preparation method and application thereof
A cerium-zirconium-aluminum, solid solution technology, applied in the direction of separation methods, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of unsatisfactory catalytic performance of non-precious metal catalysts, and solve the problem of difficult to improve degradation performance and good catalytic activity , increasing the effect of oxygen vacancies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
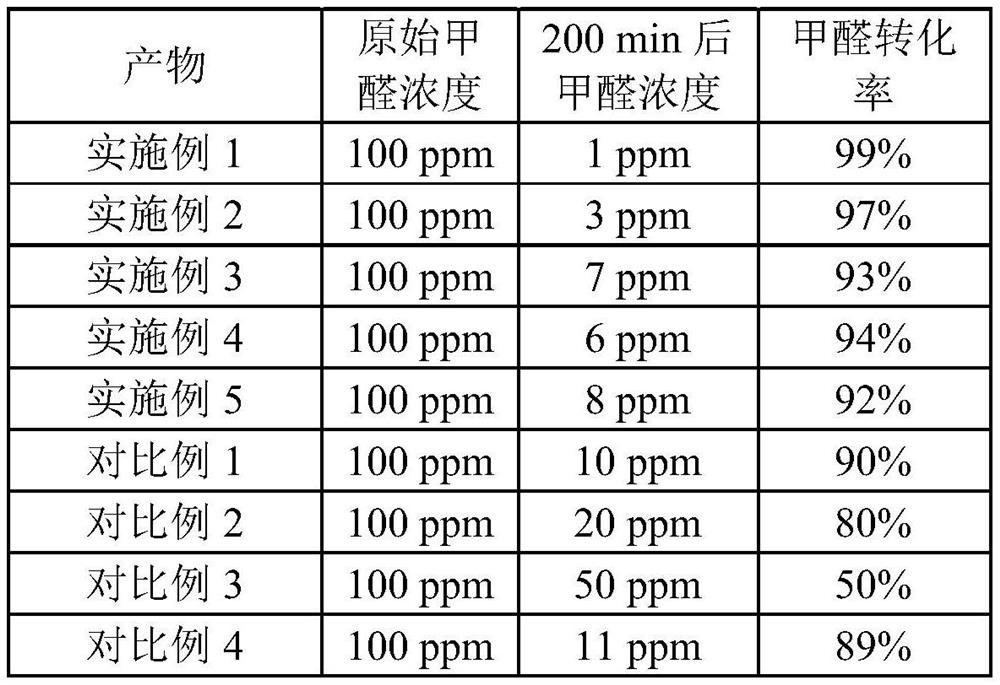
Embodiment 1
[0029] A chromium-cerium-zirconium-aluminum solid solution material, the specific preparation process of which is as follows:
[0030] (1) Obtaining the reaction bottom solution: Weigh 1 g of P123 and dissolve it in 20 mL of absolute ethanol at room temperature, stir magnetically at 1000 rpm, slowly add 1.5 mL of 67% nitric acid, and stir for 30 min.
[0031] (2) 3.8 g of aluminum nitrate was added to the reaction bottom liquid, and after stirring for 30 minutes, cerium acetate, zirconium chloride, and chromium chloride were added in sequence, and the reaction initial liquid was obtained by continuing to stir for 6 hours at room temperature. Wherein, the addition amount of the cerium precursor is 3wt% of the mass of the aluminum precursor, the addition amount of the zirconium precursor is 3wt% of the mass of the aluminum precursor, and the addition amount of the chromium precursor is 5wt% of the mass of the aluminum precursor.
[0032] (3) The initial reaction solution was tra...
Embodiment 2
[0035] A chromium-cerium-zirconium-aluminum solid solution material, the specific preparation process of which is as follows:
[0036] (1) Acquisition of reaction bottom liquid: Weigh 0.6g of P123 and dissolve it in 20mL of absolute ethanol at room temperature, stir magnetically at 1000rpm, slowly add 1.4mL of 67% nitric acid, and stir for 30min.
[0037] (2) 2 g of aluminum isopropoxide was added to the above reaction bottom liquid, and after stirring for 30 minutes, cerium chloride, zirconium nitrate, and chromium nitrate were added in sequence, and the reaction initial liquid was obtained by continuing to stir at room temperature for 6 hours. Wherein, the addition amount of cerium precursor is 3wt% of the mass of aluminum precursor, the addition amount of zirconium precursor is 1wt% of the mass of aluminum precursor, and the addition amount of chromium precursor is 5wt% of the mass of aluminum precursor.
[0038] (3) The initial reaction solution was transferred to a petri ...
Embodiment 3
[0041] A chromium-cerium-zirconium-aluminum solid solution material, the specific preparation process of which is as follows:
[0042] (1) Obtaining the reaction bottom solution: Weigh 1.2 g of P123 and dissolve it in 20 mL of absolute ethanol at room temperature, stir magnetically at 1000 rpm, slowly add 1.6 mL of 67% nitric acid, and stir for 30 min.
[0043] (2) 3.4 g of aluminum sulfate was added to the above reaction bottom liquid, and after stirring for 30 minutes, cerium nitrate, zirconium chloride and chromium nitrate were added in sequence, and the initial reaction liquid was obtained by stirring continuously for 6 hours at room temperature. Among them, the addition amount of cerium precursor is 3wt% of the mass of aluminum precursor, the addition amount of zirconium precursor is 3wt% of the mass of aluminum precursor, and the addition amount of chromium precursor is 9wt% of the mass of aluminum precursor.
[0044] (3) The initial reaction solution was transferred to ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap