Method for removing ammonia nitrogen from hydrazine hydrate reduced liquid
A technology of hydrazine hydrate and ammonia nitrogen, applied in chemical instruments and methods, water pollutants, water/sewage multi-stage treatment, etc., can solve problems such as harsh working environment and inability to meet industrial wastewater discharge requirements, and achieve short reaction time and high dosage The effect of small size and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
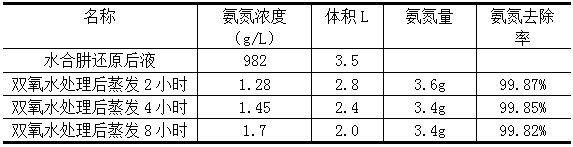
[0010] (1) Take 3.5L of palladium refining hydrazine hydrate reduction liquid wastewater, measure the ammonia nitrogen concentration to 982g / L, adjust the pH to 9, add 400ml of hydrogen peroxide with a mass fraction of 10%, and pretreat at 85 °C for 1 h. The reaction was vigorously exothermic and a large amount of white foam was produced.
[0011] (2) After the reaction is stable, the pH of the solution is adjusted to 11 with sodium hydroxide solution. Put it on an electric hot plate at 80°C for micro-boiling evaporation. After 2-8 hours of evaporation, filter the residue to separate the liquid. The removal rate of ammonia nitrogen is measured as shown in the table below, and the filtrate is discharged to the standard.
[0012] Processing effect
[0013]
Embodiment 2
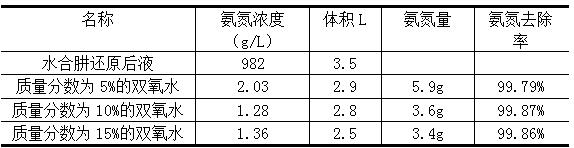
[0015] (1) Take 3.5L of palladium refining hydrazine hydrate reduction liquid wastewater, measure the ammonia nitrogen concentration to 982g / L, adjust the pH to 9, add 400ml of hydrogen peroxide with a mass fraction of 5%-15%, and pretreat at 85 °C for 1 h. The reaction was exothermic and a white foam was produced.
[0016] (2) After the reaction is stable, the pH of the solution is adjusted to 11 with sodium hydroxide solution. It was placed on an electric hot plate at 80°C for micro-boiling evaporation. After 2 hours of evaporation, the residue was separated by filtration. The removal rate of ammonia nitrogen was measured as shown in the table below, and the filtrate was discharged to the standard.
[0017] Processing effect
[0018]
Embodiment 3
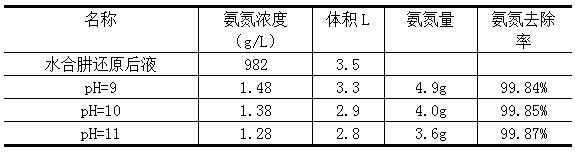
[0020] (1) Take 3.5L of palladium refining hydrazine hydrate reduction liquid wastewater, measure the ammonia nitrogen concentration to 982g / L, adjust the pH to 9-11, add 400ml of hydrogen peroxide with a mass fraction of 10%, and pretreat at 85 °C for 1 h. The reaction was vigorously exothermic and a white foam developed.
[0021] (2) After the reaction is stable, the pH of the solution is adjusted to 11 with sodium hydroxide solution. It was placed on an electric hot plate at 80°C for micro-boiling evaporation. After 2 hours of evaporation, the residue was separated by filtration. The removal rate of ammonia nitrogen was measured as shown in the table below, and the filtrate was discharged to the standard.
[0022] Processing effect
[0023]
PUM
| Property | Measurement | Unit |
|---|---|---|
| clearance rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap