Method for synthesizing 5-amino-1-pentanol by taking furfural as initial raw material
A technology of furfural and amino groups, applied in the field of synthesizing 5-amino-1-pentanol, can solve the problems of high energy consumption, low efficiency, high price and the like, and achieve the effects of simple preparation process, good catalytic performance and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
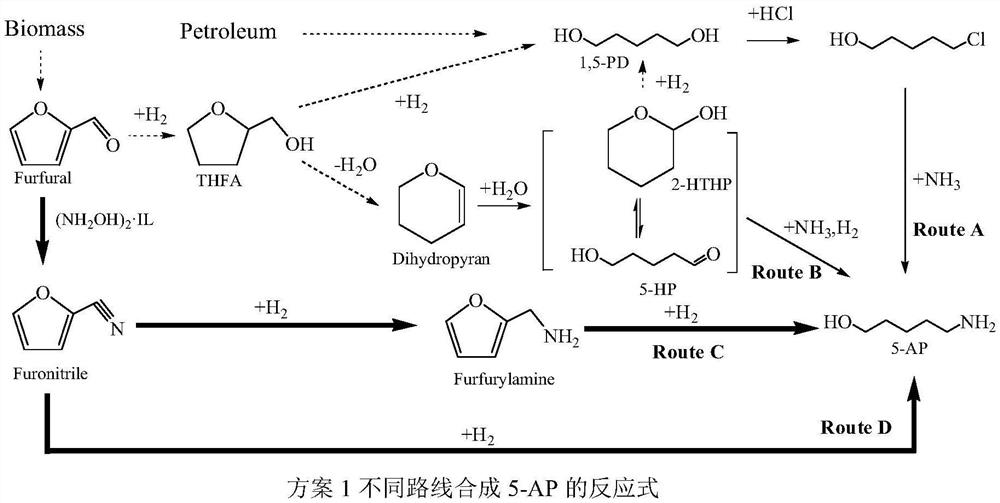
[0063] Step 1. Add furfural (3.6mmol), N,N,N-trimethyl-N-sulfobutylammonium hydrogen sulfate ionic liquid hydroxylamine salt (5.4mol) and zinc chloride (0.144mmol) into a 100ml three-necked flask 6ml of p-xylene and 6ml of N,N,N-trimethyl-N-sulfobutylammonium hydrogen sulfate ionic liquid were added to the mixture, stirred, refluxed and condensed, and the reaction was stopped after 8 hours of reaction at normal pressure and 100°C. After the reaction solution was cooled, the supernatant was taken and analyzed by gas chromatography. The reaction result showed that the conversion rate of furfural was 100%, and the yield of furfural was 99%. The toluene layer was taken, and the solvent was distilled off under reduced pressure to obtain high-purity furfuronitrile.
[0064] Step 2. Put 2 mmol of furonitrile, 0.1 g of Ru / g-C with a loading of 5% 3 N 4 The catalyst, 20 mL of isopropanol, and 500 μL of ammonia water (25 wt %) were added to a 50 mL autoclave. First, the air in the rea...
Embodiment 2
[0070] The other parts of step 1 are the same as in Example 1, the difference is that the added N,N,N-trimethyl-N-sulfobutylammonium hydrogen sulfate ionic liquid hydroxylamine salt is 4.32mmol, and the reaction result is that the furfural conversion rate is 69.2% , the yield of furfuronitrile was 66.7%. The three-step series reaction is required to be carried out under the optimal conditions for the first two-step reaction. When the first two-step reaction yield does not reach 99%, there are many by-products after removing the solvent, so the subsequent steps 2 and 3 are not carried out.
Embodiment 3
[0072] The other parts of step 1 are the same as in Example 1, the difference is that the N,N,N-trimethyl-N-sulfobutylammonium hydrogen sulfate ionic liquid hydroxylamine salt added is 4.68mmol, and the reaction result is a furfural conversion rate of 78.5% , the yield of furfuronitrile was 75.2%. The three-step series reaction is carried out under the optimal conditions of the first two-step reaction. When the first two-step reaction yield does not reach 99%, there are many by-products after removing the solvent, so the subsequent steps 2 and 3 are not carried out.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com