Melatonin-platinum (IV)-carbon nitrogen long-chain complex, preparation method and application of melatonin-platinum (IV)-carbon nitrogen long-chain complex in tumor drugs
An anti-tumor drug, melatonin technology, applied in the application, preparation, and field of melatonin-platinum-carbon-nitrogen long-chain complexes in tumor drugs, can solve the problem of concentrated effect time and improve the body's immunity Power, less side effects, the effect of regulating biological rhythm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0072] Wherein, the preparation method of the compound of formula 21 comprises:
[0073] The melatonin of formula 24 and the compound of formula 25 (succinic anhydride or glutaric anhydride) are subjected to acylation reaction in the presence of the second condensing agent and the second acid binding agent to obtain the compound of formula 11;
[0074]
[0075] Wherein, the second condensing agent is DMAP; the second acid binding agent is triethylamine.
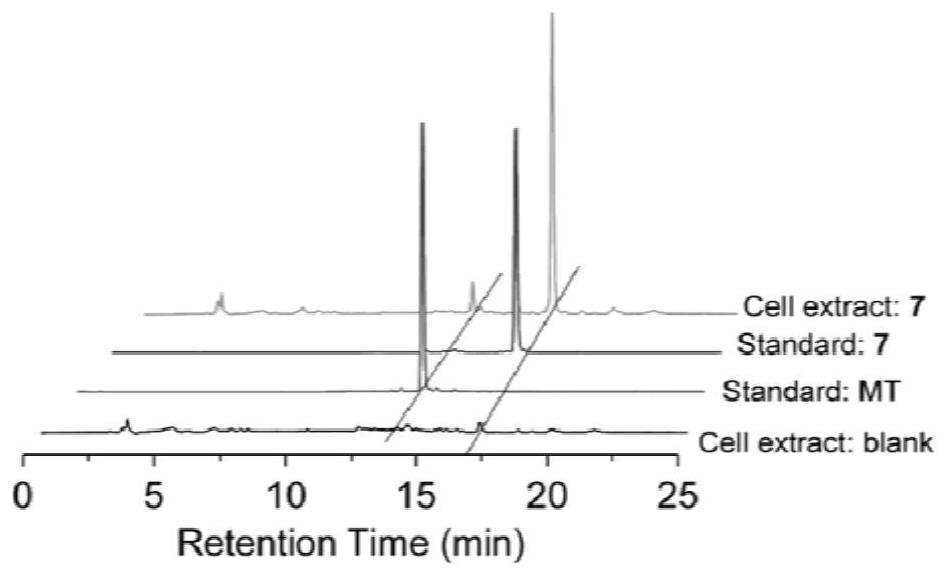
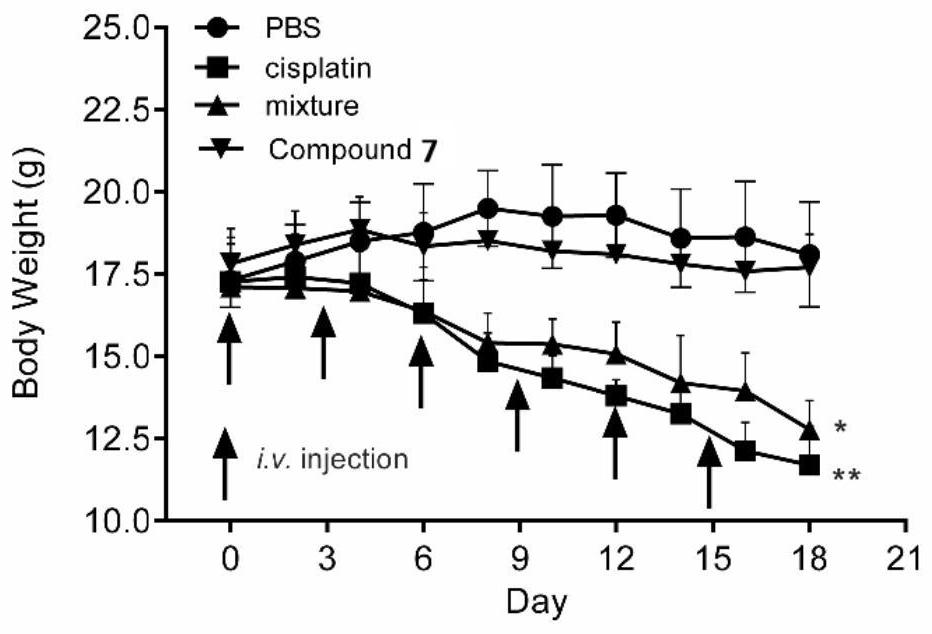
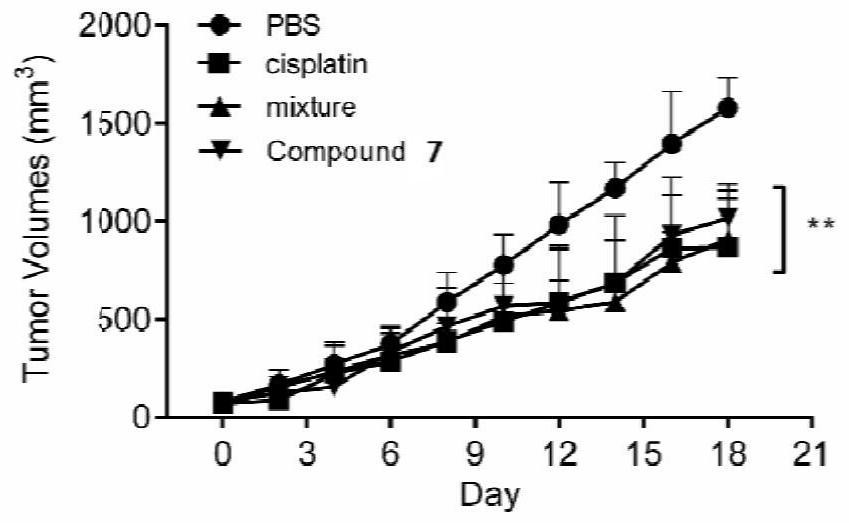
[0076] The above-mentioned melatonin-platinum (IV)-carbon-nitrogen long-chain complex can be used to prepare anti-tumor drugs, for example, can be used to prepare anti-ovarian cancer, cervical cancer, breast cancer, lung cancer, liver cancer, intestinal and gastric cancer drugs; especially suitable for sex hormones Related tumors ovarian cancer, cervical cancer, breast cancer drugs.
Embodiment 1
[0079] The structural formula of the melatonin-platinum (IV)-carbon nitrogen long-chain complex a of this embodiment is as follows:
[0080]
[0081] The synthesis route of the preparation method of the melatonin-platinum (IV)-carbon nitrogen long-chain complex described in this embodiment is as follows:
[0082]
[0083]
[0084] In step 1, cisplatin was oxidized under the condition of hydrogen peroxide for 6 h, and after the end, it was refrigerated at 0-4 °C overnight, and then washed with water, ice ethanol and ether to obtain a pale yellow precipitate Oxoplatin (c,c,t-[ Pt(NH 3 ) 2 Cl 2 (OH) 2 ]), a1.
[0085] Step 2, melatonin (MT) is reacted with succinic anhydride, 4-dimethylaminopyridine (DMAP) is added in the reaction, triethylamine (Et 3 N), protected with argon during the reaction, the reaction temperature was 50 °C for 48 h, the reaction solution was concentrated into oil and separated by silica gel chromatography column, the eluents were dichloromet...
Embodiment 2
[0090] The structural formula of the melatonin-platinum (IV)-carbon-nitrogen long-chain complex b of this embodiment is as follows:
[0091]
[0092] The route is as follows:
[0093]
[0094]
[0095] In step 1, cisplatin was oxidized under the condition of hydrogen peroxide for 6 h, and after the end, it was refrigerated at 0-4 °C overnight, and then washed with water, ice ethanol and ether to obtain a pale yellow precipitate Oxoplatin (c,c,t-[ Pt(NH 3 ) 2 Cl 2 (OH) 2 ]), a1.
[0096] Step 2, melatonin (MT) is reacted with glutaric anhydride, 4-dimethylaminopyridine (DMAP) is added in the reaction, triethylamine (Et 3 N), protected with argon during the reaction, the reaction temperature was 50°C for 48h, the reaction solution was concentrated into oil and separated by silica gel chromatography column, the eluents were dichloromethane and methanol, and milky white precipitate b2 was obtained.
[0097] Step 3, mix the product b2 in step 2 with dry dimethyl sulf...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap