Skin-derived fibroblast having tendon regeneration effect and use thereof
A technology for fibroblasts, tendons, applied in the field of pharmaceutical compositions for tendon regeneration and therapy, to achieve high expression levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Culture of skin-derived fibroblasts
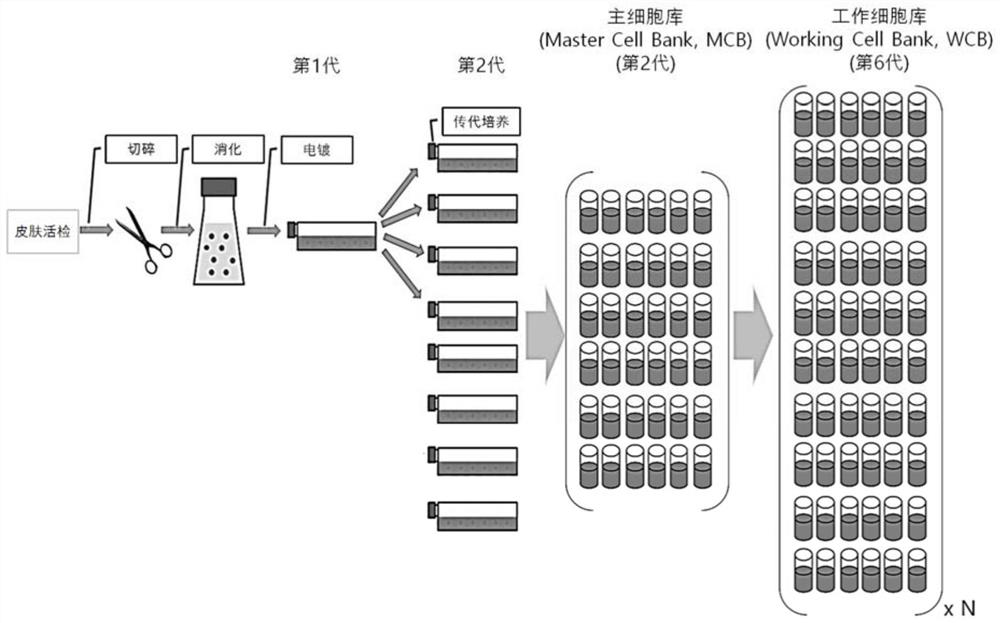
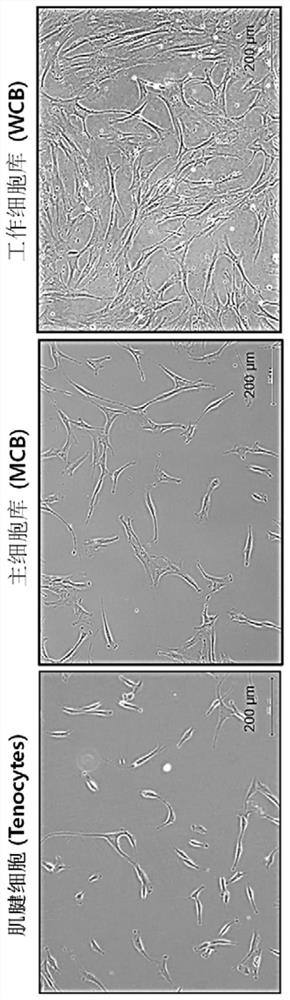
[0044] The normal skin tissue isolated from the donor was minced, and only fibroblasts were isolated by disintegration. The isolated fibroblasts were primary cultured in Dulbecco's Modified Eagle's medium (DMEM) / F12 (Invitrogen, USA) containing 10% fetal bovine serum (FBS). primary culture), the culture conditions were maintained at 37°C, 10% CO 2 humid environment. After subculturing at intervals of about 5 to 6 days, a Master Cell Bank (MCB) was established at passage 2. Then, it was further subcultured at intervals of about 5 to 6 days, and a large Working Cell Bank (WCB) was obtained in passage 6 ( figure 1 ). When the cells in the petri dish were filled to about 70 to 80%, cells were separated using Trypsin-EDTA to carry out cell subculture. In most in vitro cell cultures, 5% CO 2 Used as a gas composition, but in the present invention, 10% CO is used 2 , to keep the pH of the medium exactly at 7.4.
[0045]...
Embodiment 2
[0047] Example 2: Confirmation of properties of skin-derived fibroblasts
[0048] 2-1. Proliferation and morphological characteristics of skin-derived fibroblasts
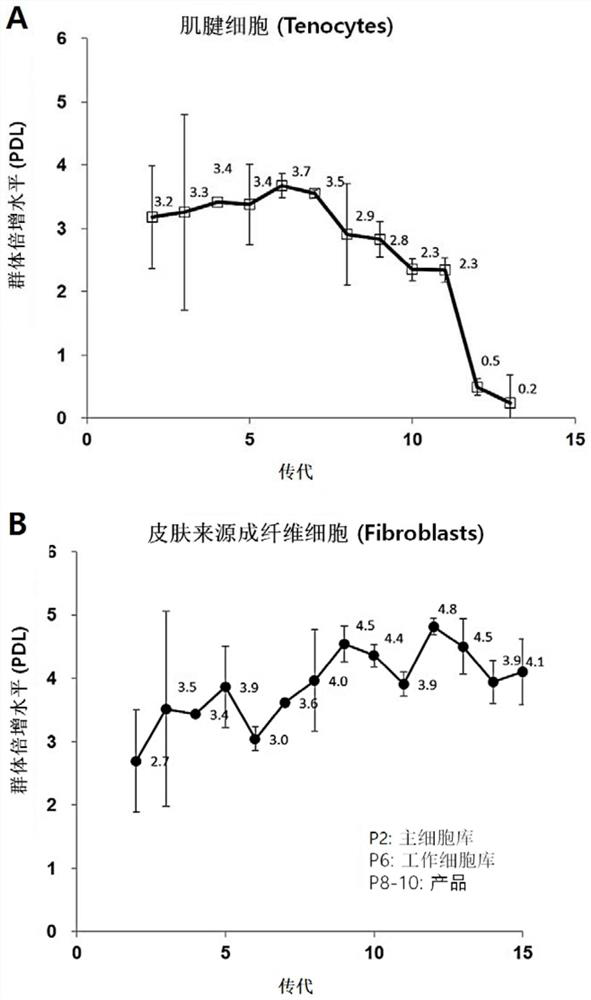
[0049] The skin-derived fibroblasts obtained in Example 1 were cultured from passage 2 to passage 10 under the same culture conditions, and the doubling time of the cells was calculated by counting the number of cells at the time of initial plating and the total number of cells after the cells were obtained (doubling time). As a result, it took an average of 30 hours from passage 2 to passage 10 and showed similar values for the two different media.
[0050] In addition, from the results of confirming the cell proliferation level (PDL) according to the following mathematical formula 1, it was calculated that the skin-derived fibroblasts obtained in Example 1 were 2.7 to 4.5 per passage ( figure 2 , lower graph; N=3, mean standard deviation). This result is in contrast to the short cell lifespan of tenocytes in...
Embodiment 3
[0064] Example 3: Confirmation of the quality of skin-derived fibroblasts
[0065] The skin-derived fibroblasts of the cryopreserved cell bank were thawed and then re-cultured to confirm the quality. As a result, the recultured skin-derived fibroblasts exhibited excellent thawing rate and cell survival rate, and it was confirmed that the cell quality was maintained regardless of the freezing period (Table 1).
[0066] Table 1
[0067]
[0068]
[0069] In addition, the results of the foreign virus negative test and the microorganism residue test that meet the cell therapy approval standards of the Korean Ministry of Food and Drug Safety have confirmed that there are no foreign viruses and microorganisms remaining in the cell bank.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com