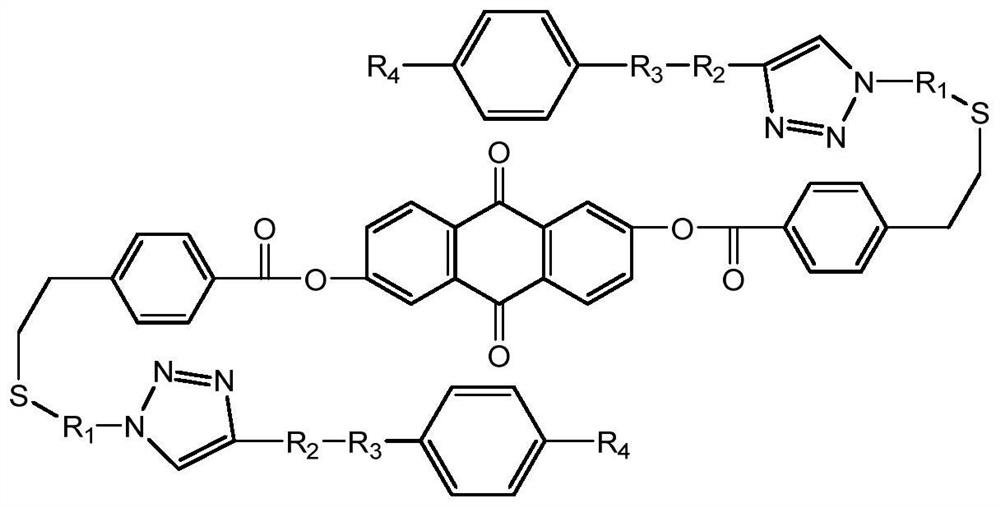
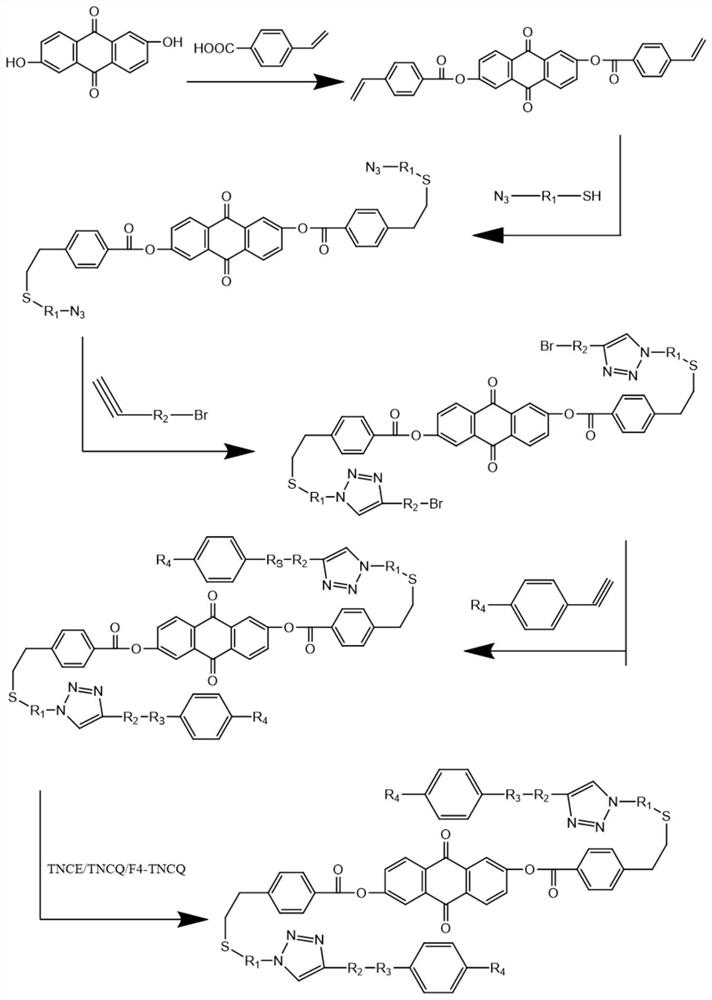
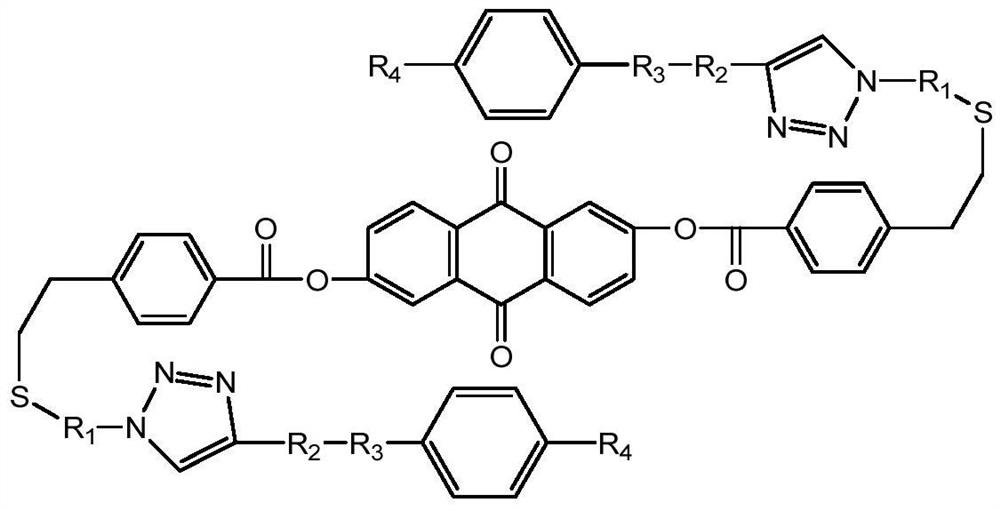
Broadband liquid crystal laser protection material prepared through multi-key click chemistry and preparation method of broadband liquid crystal laser protection material
A multi-click, laser protection technology, applied in the direction of organic chemistry, can solve the problem of weak broadband absorption capacity, etc., to achieve the effect of expanding the band range and absorption capacity, improving stability, and excellent photoelectric properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] A preparation method for preparing a broadband liquid crystal laser protective material by multiple click chemistry, comprising the following steps:
[0045] Step 1, esterification of 2,6-dihydroxyanthraquinone with 4-vinylbenzoic acid
[0046] Dissolve 0.1-20 parts of 2,6-dihydroxyanthraquinone and 0.2-40 parts of 4-vinylbenzoic acid (ratio 1:2~1:4) in 50-300mL of anhydrous dichloromethane and put in 50 -Into a 500mL flask, add 0.2-40 parts of dicyclohexylcarbodiimide and 0.01-5 parts of 4-dimethylaminopyridine, stir at room temperature for 23-26h, rotate for 10-60min, and perform silica gel column chromatography, wherein Dichloromethane was used as the eluent to obtain the sample 9,10-dioxo-9,10-dihydroanthracene-2,6-diylbis(4-vinylbenzoate), the chemical formula of the sample is as follows
[0047]
[0048] In step 2, taking 4-azidothiophenol as an example, it is introduced into the product of step 1 through a thiol double bond click reaction
[0049] Take 0.1-2...
Embodiment 1
[0074] Example 1: R1, R2 take the benzene ring as an example, the halogenated alkane uses bromobutane, and [2+2] click chemistry uses TNCE as the click reagent to make broadband laser protection materials.
[0075] Step 1: Dissolve 0.1 part of 2,6-dihydroxyanthraquinone and 0.2 part of 4-vinylbenzoic acid (ratio 1:2~1:4) in 50mL of anhydrous dichloromethane and put it into a 100mL flask , 0.2 part of dicyclohexylcarbodiimide (DCC) and 0.01-part of 4-dimethylaminopyridine (DMAP) were added to it, stirred at room temperature for 24 h, rotary evaporated for 30 min, and chromatographed on silica gel column with dichloromethane as the eluent liquid to obtain the sample 9,10-dioxo-9,10-dihydroanthracene-2,6-diylbis(4-vinylbenzoate), with a yield of 92%.
[0076] Step 2, take 0.1 part of 9,10-dioxo-9,10-dihydroanthracene-2,6-diylbis(4-vinylbenzoate) and 0.2 part of 4-azidothiophenol Put 50 mL of N,N-dimethylformamide (DMF) into a 100 mL reaction flask with 50 mL of N,N-dimethylforma...
Embodiment 2
[0084] Example 2: R1, R2 take benzene ring as an example, halogenated alkanes use bromobutane, [2+2] click chemistry uses TNCQ as click reagent to make broadband laser protection materials
[0085] Step 1: Dissolve 20 parts of 2,6-dihydroxyanthraquinone and 40 parts of 4-vinylbenzoic acid (ratio 1:2~1:4) in 300mL of anhydrous dichloromethane and put it into a 500mL flask , 0.2 part of dicyclohexylcarbodiimide (DCC) and 0.01-part of 4-dimethylaminopyridine (DMAP) were added to it, stirred at room temperature for 24 h, rotary evaporated for 30 min, and chromatographed on silica gel column with dichloromethane as the eluent liquid, to obtain the sample 9,10-dioxo-9,10-dihydroanthracene-2,6-diylbis(4-vinylbenzoate) with a yield of 91%.
[0086] Step 2, take 20 parts of 9,10-dioxo-9,10-dihydroanthracene-2,6-diylbis(4-vinylbenzoate) and 40 parts of 4-azidothiophenol Put 300 mL of N,N-dimethylformamide (DMF) into a 500 mL reaction flask with 300 mL of N,N-dimethylformamide (DMF) as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com