Method for removing bacterial endotoxin in low-molecular-weight heparin
A technology of low molecular weight heparin and bacterial endotoxin, applied in the field of biomedicine, to achieve the effect of easy production and amplification, simple and quick method, and thorough removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
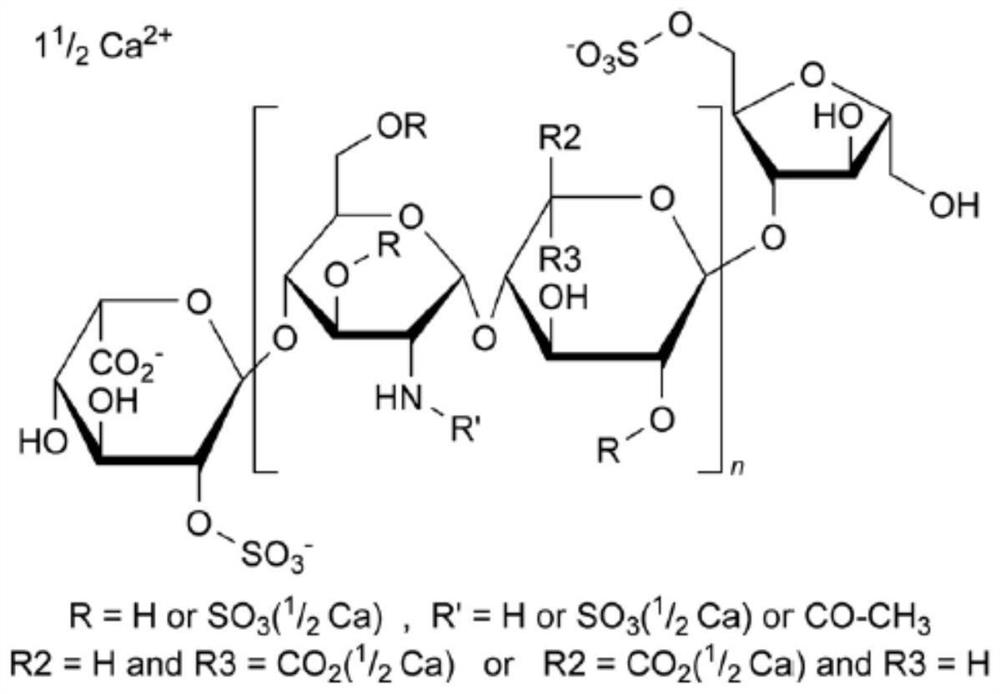
[0030] Embodiment 1, the removal method of bacterial endotoxin in nadroparin calcium
[0031] Step S1: take 1kg of sodium heparin, add 5kg of water to dissolve, adjust the pH to 2.6, add 27g of sodium nitrite for cracking for 2h, adjust the pH to neutrality, add 20g of sodium borohydride for reduction, add 3kg of calcium chloride to convert it into a calcium salt to obtain Nadroparin calcium crude solution, the volume is about 6L;
[0032] Step S2: add 1.2L n-butanol-butyl acetate mixed solution to the nadroparin calcium crude product solution in step S1, the volume ratio of n-butanol and butyl acetate in the n-butanol-butyl acetate mixed solution is 7 : 1, stirred for 20min, left standstill for 40min, discarded the upper organic phase, and repeated extraction with n-butanol-butyl acetate mixed solution, a total of 5 times, n-butanol and acetic acid in the n-butanol-butyl acetate mixed solution The volume ratio of butyl ester is 7:1, and the organic phase is discarded. At thi...
Embodiment 2
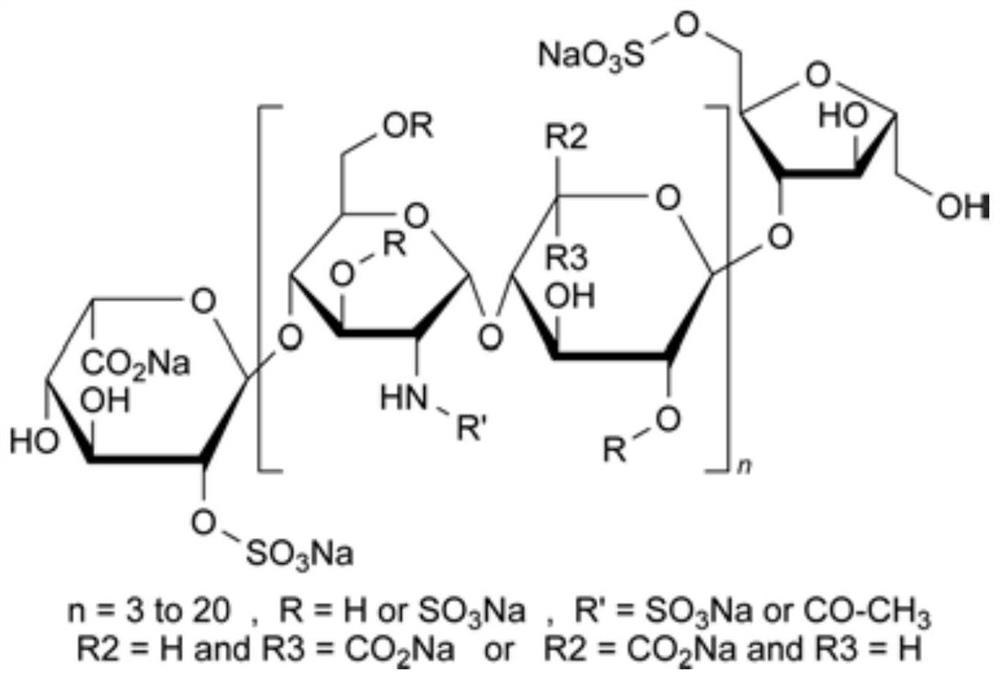
[0034] Embodiment 2, the removal method of bacterial endotoxin in dalteparin sodium
[0035] Step S1: take 1kg of sodium heparin, add 5kg of water to dissolve, adjust the pH to 2.6, add 22g of sodium nitrite to crack for 2h, adjust the pH to neutrality, add 16g of sodium borohydride for reduction, and obtain a crude heparin sodium solution with a volume of about 6L;
[0036] Step S2: add 1.8L n-butanol-butyl acetate mixed solution to the dalteparin sodium crude product solution in step S1, the volume ratio of n-butanol and butyl acetate in the described n-butanol-butyl acetate mixed solution is 10: 1. Stir for 30 minutes, let stand for 60 minutes, discard the upper organic phase, and repeat the extraction with n-butanol-butyl acetate mixed solution for a total of 4 times. In the n-butanol-butyl acetate mixed solution, n-butanol and butyl acetate The volume ratio of the ester is 10:1, and the organic phase is discarded. At this time, most of the bacterial endotoxin has been rem...
Embodiment 3
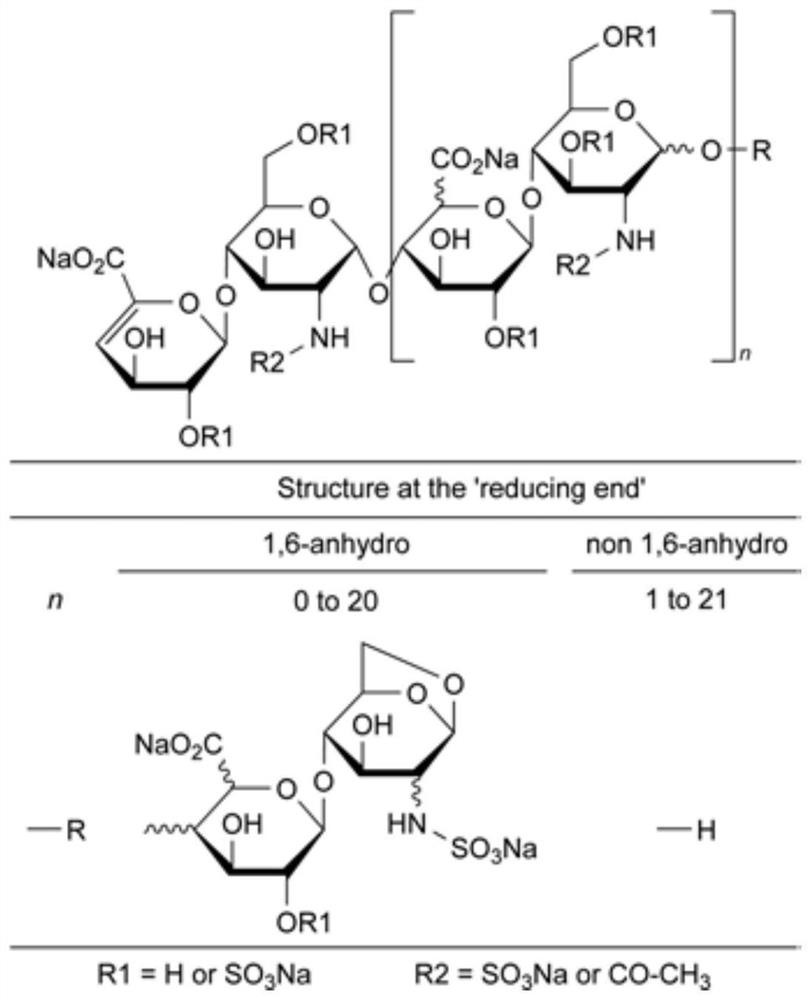
[0038] Embodiment 3, the removal method of bacterial endotoxin in enoxaparin sodium
[0039]Step S1: take 1kg of sodium heparin, dissolve it in 10kg of water, add it to 12.5kg and 20% benzethonium chloride, stir and heat up to 55°C for 2h, cool down, filter and dry to obtain 3.1kg of ammonium salt; Dissolve an appropriate amount of N,N-dimethylformamide, heat up to 50°C, add benzyl chloride for esterification for 20h, then cool down, precipitation in alcohol, filter, and dry to obtain 1.2kg of esterified product; dissolve the esterified product with appropriate amount of water, heat up To 60 ℃, add an appropriate amount of lye for cracking, cooling, and oxidation to obtain a crude enoxaparin sodium solution with a volume of about 9.5L;
[0040] Step S2: Add 1.9L of n-butanol-butyl acetate mixed solution to the enoxaparin sodium crude product solution in step S1, and the volume ratio of n-butanol to butyl acetate in the n-butanol-butyl acetate mixed solution is 9 : 1, stir for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com