Method for regulating and controlling conduction velocity of tissue-engineered conduction bundle and application
A tissue engineering and conduction velocity technology, applied in the field of biomedical engineering, can solve problems such as slowing myocardial conduction velocity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
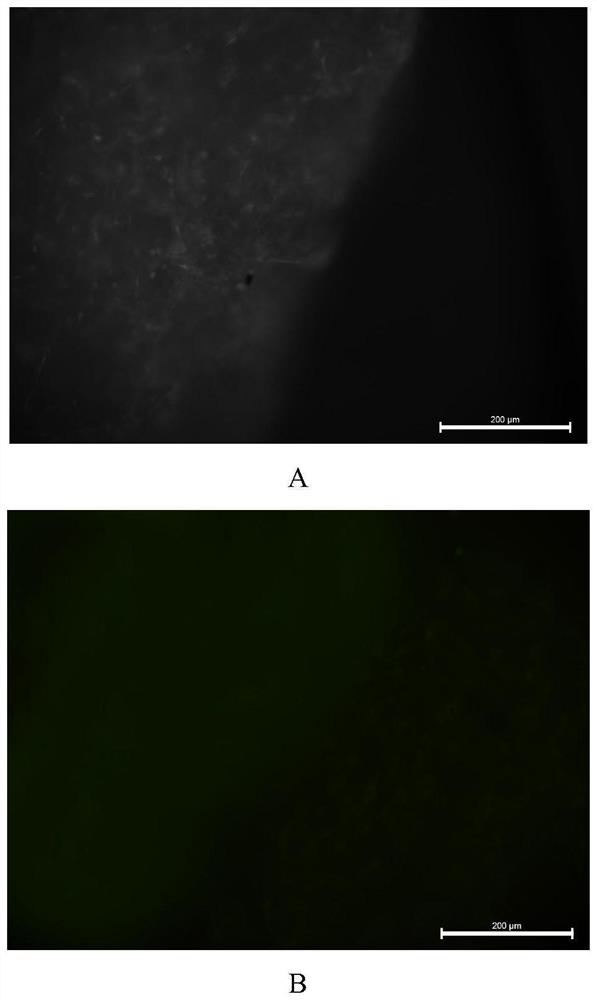
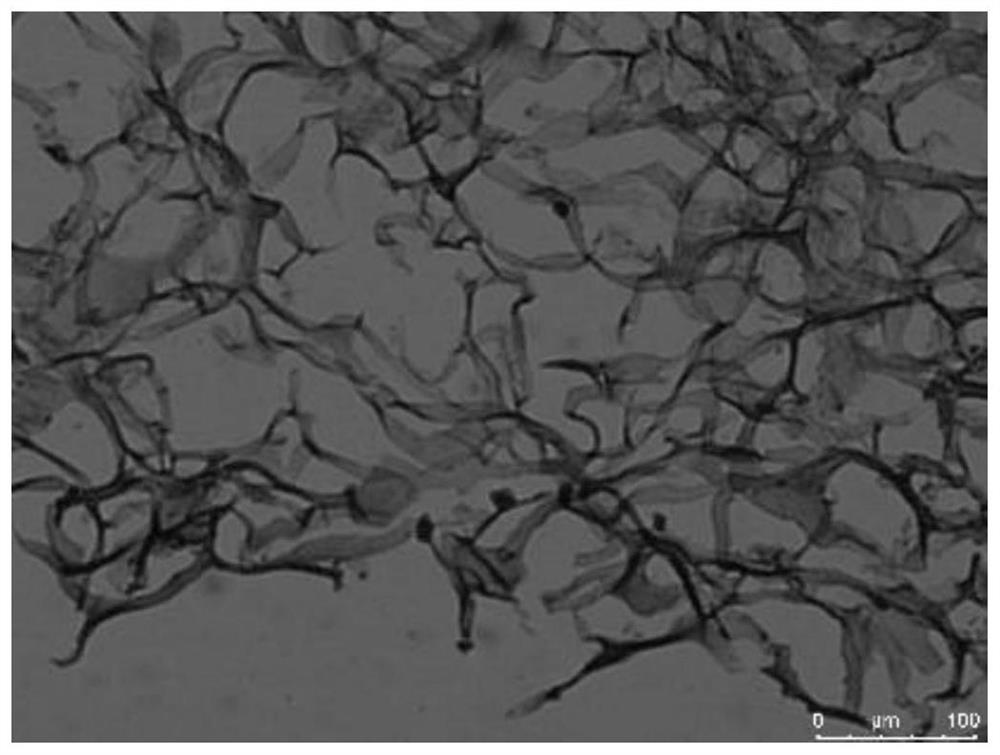
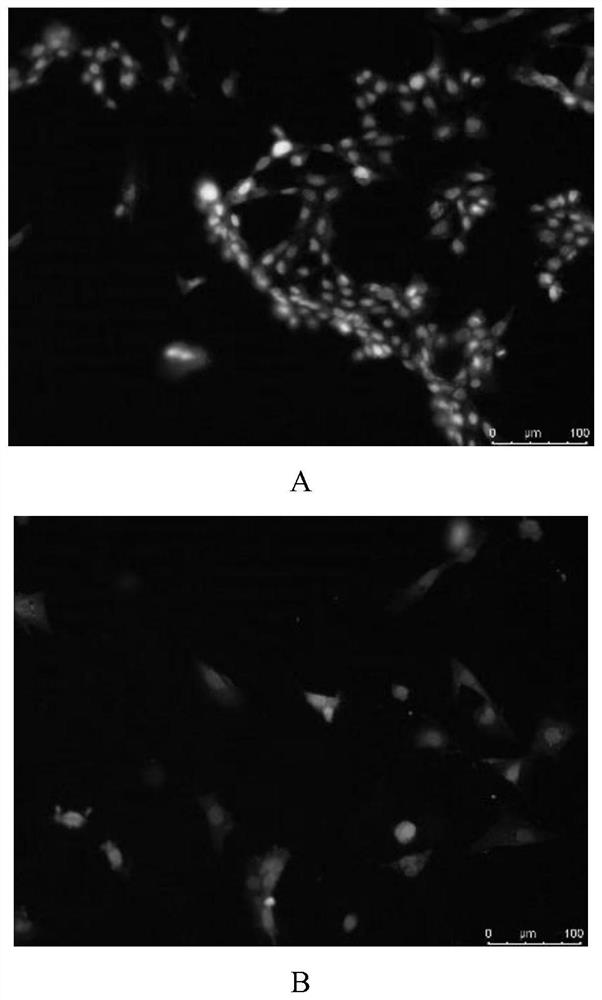
[0044] Example 1. Screening of the regulation scheme of rat ECT conduction velocity
[0045] 1. TBX3 transfected H9C2 cells
[0046] (1) Design PCR primers, extract total RNA from rat embryos, amplify Tbx3 cDNA, insert Tbx3 cDNA into pLenti-EF1-EGFP-P2A-Puro-CMV-3Flag-WPRE to obtain pLenti-EF1-EGFP-P2A- The Puro-CMV-TBX3-3Flag-WPRE recombinant vector system was co-cultured with pHelper1.0 and pHelper2.0;
[0047] (2) Discard the original medium in the culture bottle, add 3ml PBS to wash three times, add 3ml trypsin and place in a 37°C constant temperature incubator to digest for 3min;
[0048] (3) Observe under the microscope, when the digestion is complete, mix with a pipette, add 20ul to the cell counting plate, and count with a cell counter;
[0049] (4) Centrifuge with a centrifuge, discard the supernatant, add 1 ml of complete medium and mix well, and pipette the cell suspension into a 6-well plate, so that the number of cells in each well is 10 6 indivual;
[0050] (...
Embodiment 2
[0078] Example 2: Verification of the effect of ECT in vivo transplantation after conduction velocity regulation
[0079] The ECT successfully constructed in the above example 1 was transplanted into the subepicardium at the atrioventricular junction of rats (Experimental Animal Center, Second Military Medical University, 250 g) through thoracotomy, that is, the ECT was connected to the atrioventricular sulcus on both sides of the heart. In myocardium; cyclosporine A (Beijing Shuanglu Pharmaceutical Co., Ltd., dose of 0.25 mg / day) and prednisolone (Shanghai General Pharmaceutical Co., Ltd., dose of 0.1 mg / day were started 3 days before transplantation ) to suppress immune rejection.
[0080] Animals were anesthetized for 6 weeks after transplantation, atrioventricular block was performed, and electrocardiogram monitoring was performed to verify conduction velocity that produced physiological atrioventricular delay. ECT was adjusted using the obtained parameters, and the adjus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com