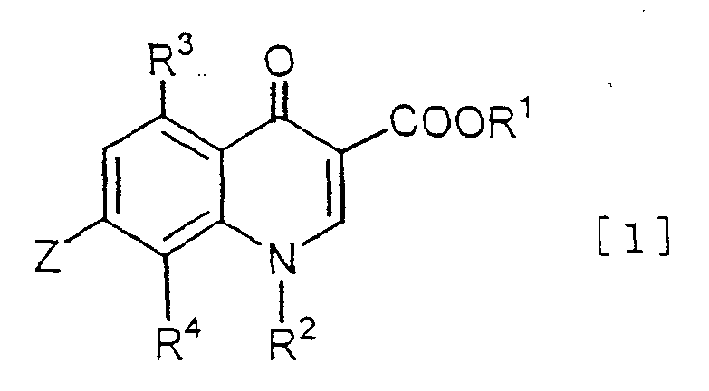
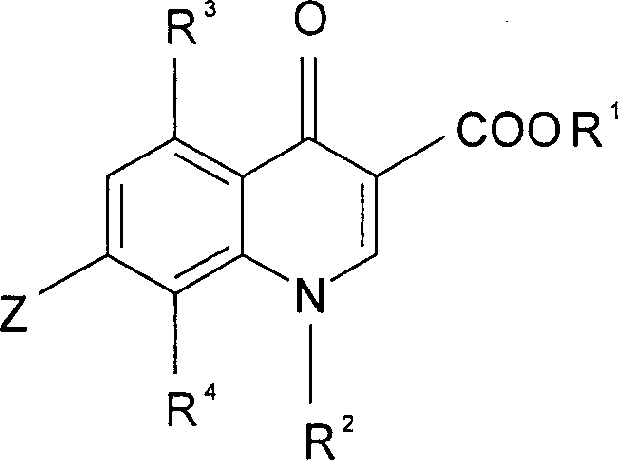
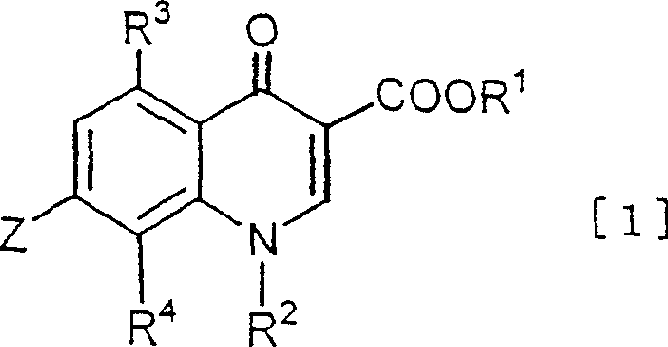
Quinolonecarboxylic acid derivatives or salts thereof
A technology of quinolone carboxylic acid and derivatives, applied in the field of novel quinolone carboxylic acid derivatives and their salts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0390]12.24g of 2,6-dibromotoluene was dissolved in 120ml of toluene, and 2.94g of cyclopropylamine, 0.45g of tris(dibenzylideneacetone)dipalladium, 0.91 g of (S)-(-)-2,2'-bis(diphenylphosphino)-1,1'binaphthyl and 6.12 g of tert-butoxysodium (Original text: natrium tert-butokisid). The mixed solution was stirred at 80°C for 1 hour under the atmosphere of argon. After cooling the reaction mixture, 210 ml of ice water and 60 ml of ethyl acetate were added, and the pH was adjusted to pH 1 with 6 mol / mL hydrochloric acid. The insoluble matter was filtered off, and the organic layer was extracted. The obtained organic layer was washed with saturated brine and dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure. The resulting residue was purified by silica gel column chromatography [hexane:toluene=2:1] to obtain 9.31 g of N-cyclopropyl-3-bromo-2-methylaniline as a pale yellow oil.
[0391] IR (pure) cm -1 :3420
[0392] NMR (CDCl 3 ...
reference example 2
[0399] 0.68 g of N-cyclopropyl-3-bromo-2-methylaniline was dissolved in 0.65 g of diethyl ethoxymethylene malonate, and stirred at 130° C. for 4 hours. After distilling off generated methanol, 4.07 g of polyphosphoric acid was added, followed by stirring at 130° C. for 25 minutes. 20 ml of freezing point chloroform and 20 ml of water were added to the reaction mixture, and the organic layer was extracted. The extracted organic layer was dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure. The resulting residue was purified by silica gel column chromatography [toluene:ethyl acetate=4:1] to obtain 0.47 g of 7-bromo-1-cyclopropyl-8-methyl-4 as a pale yellow crystal -Ethyl carbonyl-1,4-dihydro-3-quinolinecarboxylate.
[0400] IR(KBr)cm -1 : 1683, 1636
[0401] NMR (CDCl 3 )δ value: 0.8-1.6 (7H, m), 2.86 (3H, s), 3.8-4.2 (1H, m), 4.39 (2H, q, J = 7.2Hz), 7.60 (1H, d, J = 8.4 Hz), 8.16(1H, d, J=8.4Hz), 8.67(1H, s)
[0402] Melting ...
reference example 3
[0408] With 4.76g of 3-bromo-5-(cyclopropylamino)-4-methylbenzoic acid tert-butyl ester and 4.7g of ethoxymethylene malonate diethyl ester at 130°C Stir for 8 hours. After the generated ethanol was distilled off under reduced pressure, it was purified by silica gel column chromatography [hexane: ethyl acetate = 4: 1] to obtain 5.09 g of 2-{[3-bromo-5-(tert. -Butylcarboxy)cyclopropyl-2-toluidineamino]methylene}malonate diethyl.
[0409] NMR (CDCl 3 )δ value: 0.6-0.9 (4H, m), 1.1-1.4 (6H, m), 1.59 (9H, s), 2.34 (3H, s), 3.1-3.3 (1H, m), 3.6-4.3 (4H , m), 7.6-7.7 (2H, m), 8.1-8.2 (1H, m)
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com