Killer cell antibiosis polypeptide activated by lymphokine and its application
An antibacterial polypeptide and α-helix technology, applied in the field of genetic engineering, can solve the problem that the characteristics of small molecule polypeptides are not clearly defined and can not be applied.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, the isolation culture of human LAK cell
[0034] Human peripheral blood mononuclear lymphocytes were isolated according to the common experimental methods of immunology. Dilute the white blood cell suspension with an equal volume of PBS, slowly add it to the lymphocyte separation medium along the wall of the centrifuge tube, and centrifuge at 2000r / min for 30min with a horizontal centrifuge. After centrifugation, milky white turbidity can be seen at the interface of the upper and middle layers layer of mononuclear cells. Aspirate the mononuclear cells in the interface layer with a capillary tube, add 5 times the volume of PBS to wash the cells by centrifugation, and repeat twice. Finally, the cell pellet was filled with complete RPMI1640 medium (containing r-IL 2100U / ml, PHA100μg / ml) was diluted to a concentration of 2×10 6 / ml for culture, the medium was changed once after three days, and the cells were collected by centrifugation after seven days. If...
Embodiment 2
[0035] Embodiment 2, the preparation of human LAK cell acid-soluble extract
[0036] Add an appropriate amount of 5% acetic acid to the cell pellet and homogenize it electrically in an ice bath. The homogenate is centrifuged at 13000r / min×6min at 4°C to collect the supernatant. The pellet was homogenized repeatedly until no complete cells could be seen under the microscope. The collected supernatant was transferred into a dialysis bag with a cut-off flow rate of 3.5KD, dialyzed at 4°C for 48 hours to remove acetic acid, and freeze-dried to obtain an acid-soluble crude extract of LAK cells, which was stored at -20°C for future use.
Embodiment 3
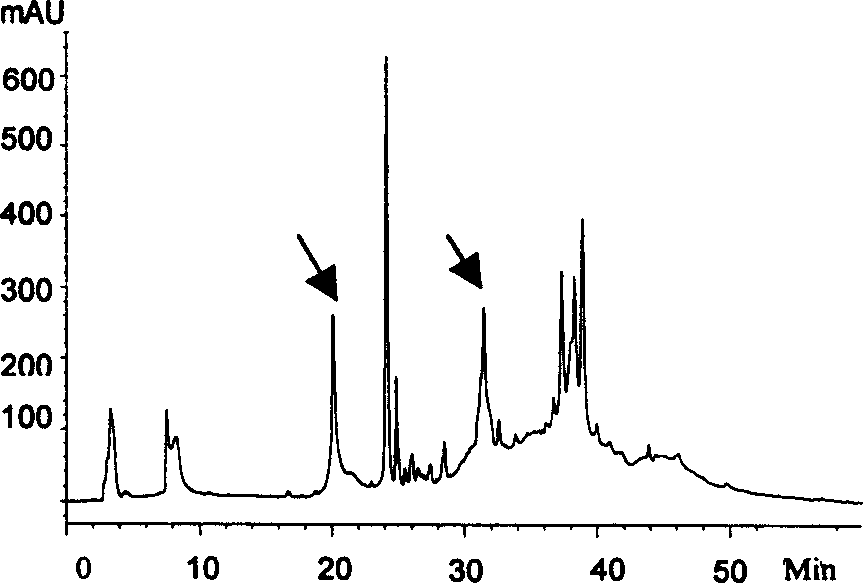
[0037] Example 3, preliminary screening of antibacterial active ingredients of human LAK cell acid-soluble extract
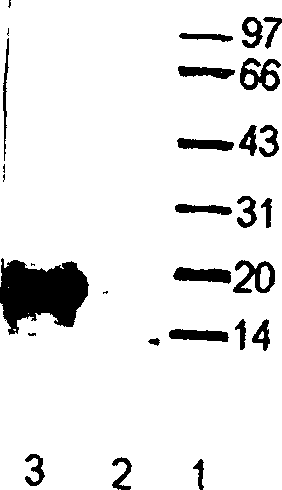
[0038] 1. Acid-Urea polyacrylamide Gel Electrophoresis (AU-PAGE)
[0039] Prepare the gel according to the Panyim method, the thickness of the gel is 0.75 mm, the gel concentration is 12.5%, and the pre-electrophoresis is performed at 150 V for 1.5 hours to remove TEMED and ammonium persulfate in the gel. Take 2 mg LAK acid crude extract, dissolve in 50 μl 0.01% acetic acid solution, and then add an equal amount of 2× loading buffer to dilute. Prepare 1 mg / ml lysozyme in the loading buffer, add 10 μl lysozyme solution and LAK crude extract solution to the gel comb wells on both sides. 150V constant voltage electrophoresis for 45min, the gel was taken out, one side was stained with Coomassie brilliant blue, and the other side was washed with 10mMPBS for 5min×3 times to remove impurities such as acetic acid and urea, and then used for sterilization experiments. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com