Active monopeptide prepared from active blood polypeptide and its prepn. activity and sequence
A technology of activity and separation process, applied in the field of active peptides and its preparation, can solve the problems of unseen separation and purification of active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] This example relates to the determination of the activity of isolated and purified samples using the Valscher respirometer manometry. details as follows:
[0072] Materials and instruments: Warburg respiration apparatus: Warburg system type 12062, produced by Braun-Melsungen, Germany; self-standardized reaction bottles without side arms; high-speed homogenizer; animals are clean-grade male guinea pigs with a weight of 250±10 g; four kinds of calves Blood deproteinized extracts are: Actovegin injection with batch number 152842 produced by Hafslund Nycomed AG in Austria; Socolseryl injection with batch number 414409 produced by Solco Basle in Switzerland; Cytopoietin with batch number 981216 produced by Jinzhou Medical College; The batch number 990102 of blood activity injection produced by Treasure Biopharmaceutical Co., Ltd. The reagents are: 10% KOH solution, 0.2mol / ml PBS and Brodie manometric fluid. The preparation method of calf blood deproteinized extract is as fo...
Embodiment 2
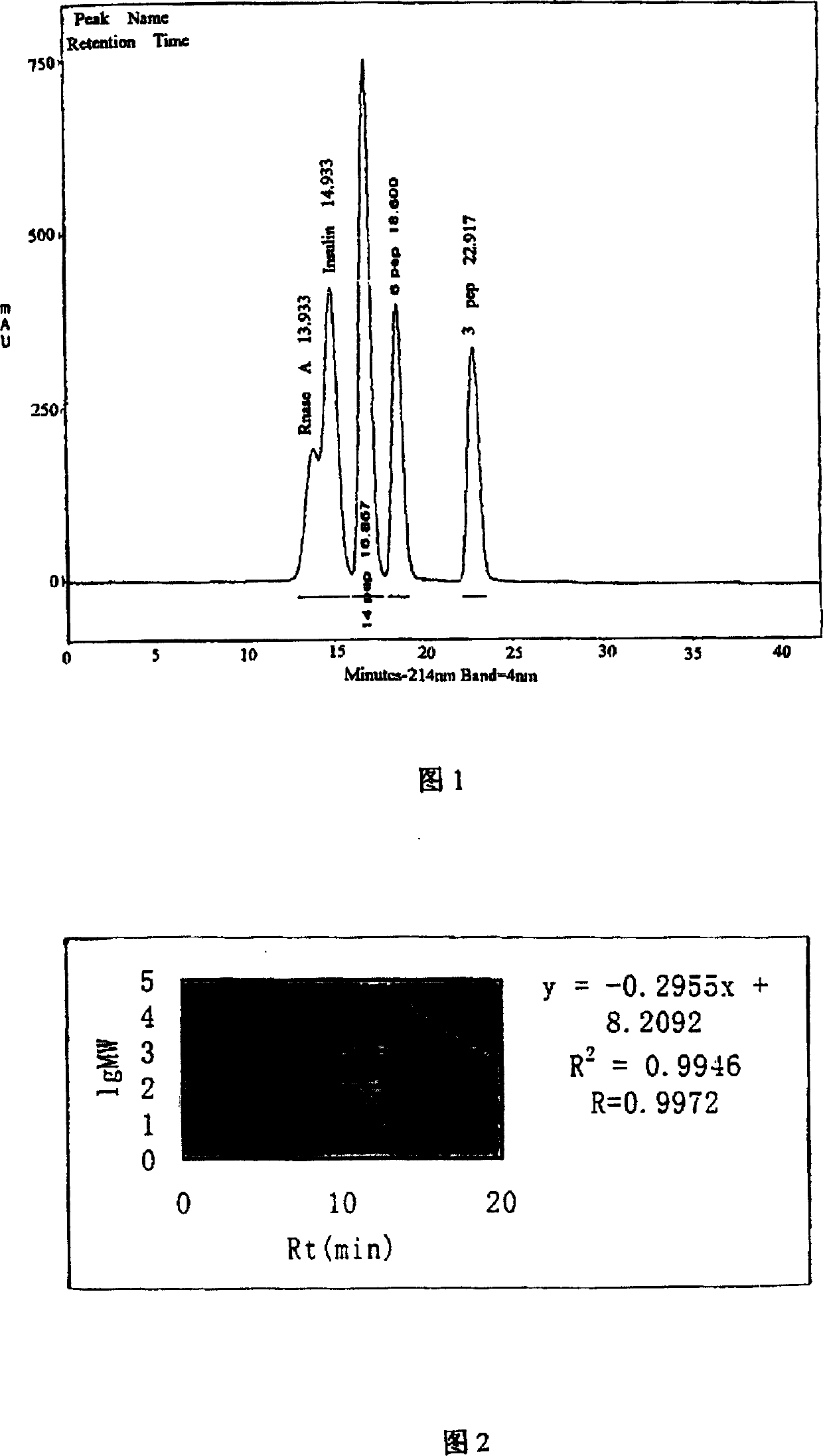
[0145] This embodiment relates to the determination of the molecular weight of Actovegin injection as a raw material for further separation and purification with high performance liquid gel chromatography (HPGC), specifically as follows:
[0146] Materials and instruments: High performance liquid chromatography: Beckman 125NM pump, 126NM detector, workstation; Superdex Peptide HR 10 / 300 column: 10mm×30cm, column volume 24ml, column packing particle size 22-44um, separation range 100-7000 channels Dayton, pressure value 1.5MPa, Pharmacia product; mobile phase: 0.05mol / L sodium phosphate+0.1mol / L sodium chloride aqueous solution, pH7.0.
[0147] The standard sample is: diribonuclease A with a relative molecular weight of 13700 Daltons, a Pharmacia product; insulin with a relative molecular weight of 5180 Daltons, a Fisherbrand product; somatostatin (14 peptides) with a relative molecular weight of 1508 Daltons; a relative molecular weight of 560 Dalton's hexapeptide, Biomol prod...
Embodiment 3
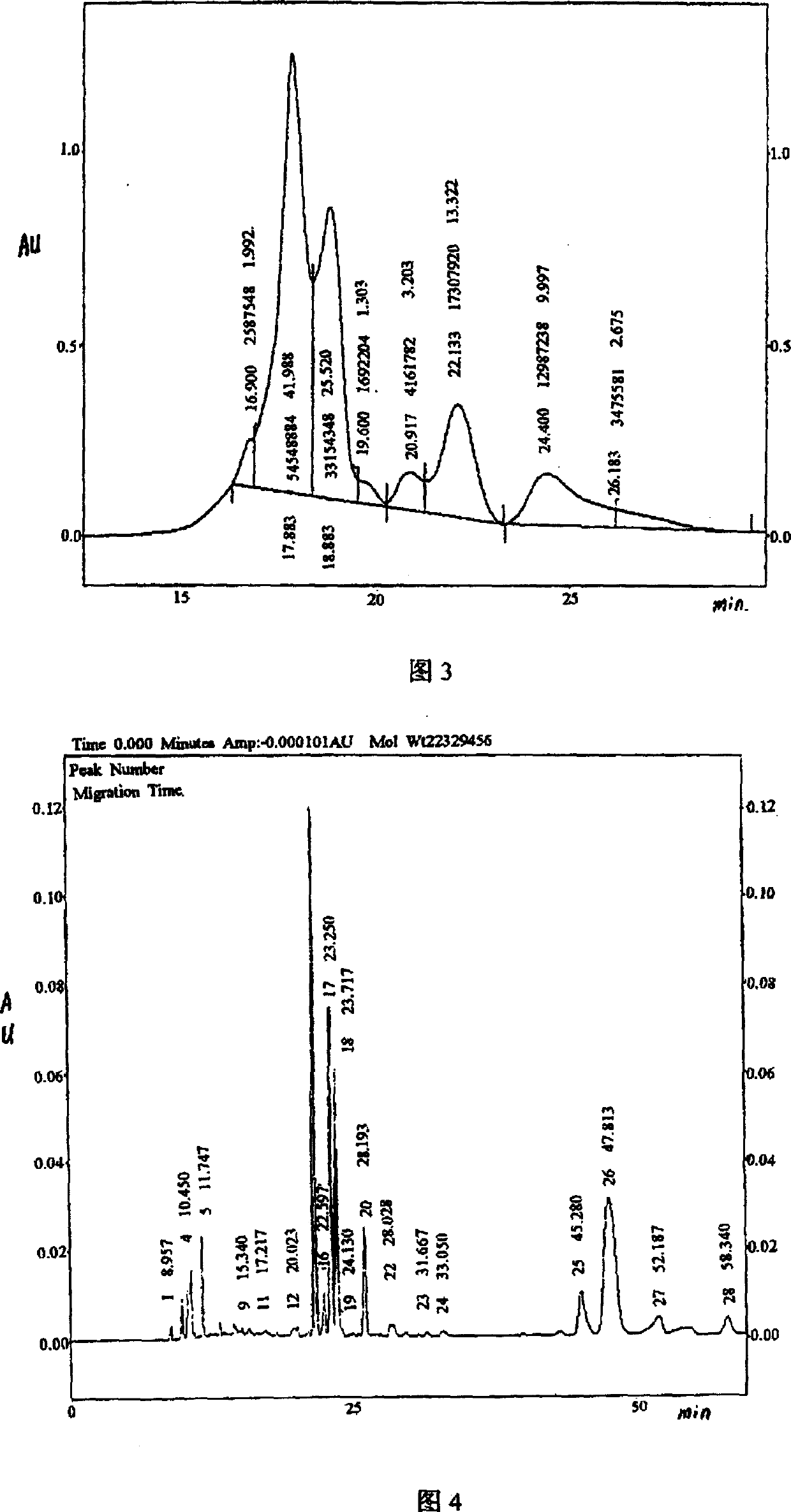
[0157] This embodiment relates to the high performance capillary zone electrophoresis (HPCZE) analysis of Actovegin injection.
[0158] Materials and instruments: Capillary electrophoresis apparatus: Beckman P / ACE 5000, fixed wavelength ultraviolet detector, workstation; Fused quartz capillary column: Beckman FS 75um * 57cm (effective length is 50cm); Sample Actovegin injection, batch number is the same as embodiment 1 ,2.
[0159] Method: prepare electrode buffer solution: take 1.7ml of 85% phosphoric acid, add 10ml of acetonitrile, add water to 250ml, 1mol / lNaOH, adjust the pH value to 2.5, filter with 0.45um membrane, and ultrasonicate before use. Before use, the capillary was washed with 1mol / l NaOH for 5 minutes, ultrapure water for 5 minutes, 0.1mol / l NaOH for 2 minutes, ultrapure water for 1 minute, and electrode buffer for 2 minutes before sample injection. The high-voltage sampling method is adopted, the sampling time is 3 seconds, the capillary sampling end is posit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com