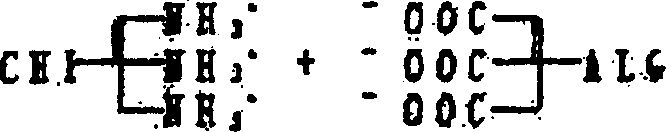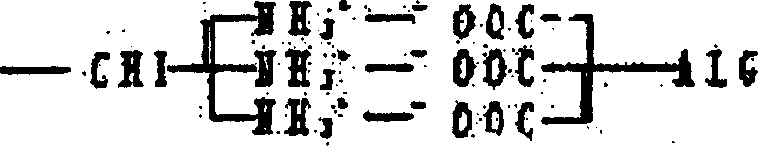Artificial biological canula and its making process
A manufacturing method and biological technology, applied in the field of biomedical materials, can solve the problems of poor resistance to normal saline, and achieve the effects of poor water resistance, excellent resistance to normal saline solubility, and prolonged absorption cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Dissolve commercially available sodium alginate with a molecular weight of 500,000 in water to obtain a high-viscosity solution with a concentration of 6% (W:W), and vacuumize and defoam until a foam-free spinning stock solution is obtained. The spinning dope is extruded from the kettle with 0.6MPa air pressure, metered by the spinning metering pump, extruded from the cortex of the hollow spinneret hole, and directly enters the coagulation bath, which is 1.5% hydrochloric acid (W:W) and 0.5 % calcium chloride (W:W) in water. At the same time, the coagulation bath was extruded from the core layer of the hollow spinneret hole with an air pressure of 0.2 MPa. The extruded spinning dope is coagulated under the simultaneous action of the external coagulation bath and the core layer coagulant, drawn out by guide rollers and drawn 1.4 times to obtain continuous alginic acid-calcium alginate hollow round tubes with an inner diameter of 0.95 mm, the thickness of the pipe wall i...
Embodiment 2
[0043] Others are the same as in Example 1, the difference is that 4wt% sodium alginate containing 7wt% (relative to the weight of sodium alginate) nerve growth factor (NGF: bFGF: compound Hongqi injection = 1: 1: 1.5 weight ratio) is used The aqueous solution is a spinning stock solution, and hollow spinneret holes with corresponding pipe diameters and wall thicknesses are used, and the coagulation bath is an aqueous solution containing 1.5% calcium chloride and 0.3% nerve growth factor. The obtained calcium alginate hollow tube was repeatedly treated three times with 0.5% dilute acetic acid solution containing 0.3% nerve growth factor (bath ratio 1:10w:w, each treatment time was 20 minutes), and the obtained drug-containing alginic acid-alginic acid The ratio of alginic acid: calcium alginate: nerve growth factor in the calcium hollow tube is 10:3.8:1, and then placed in 0.7% (W:W) of chitosan with a deacetylation degree of 82% and a molecular weight of 150,000. Growth facto...
Embodiment 3
[0045] Dissolve chitosan with a deacetylation degree of 89% and a molecular weight of 360,000 in 2% dilute acetic acid solution to obtain a solution with a chitosan concentration of 5%. The chitosan solution was added dropwise with distilled water under stirring to dilute it to 0.5% acetic acid concentration and 1.25% chitosan concentration. Add 28% nerve growth factor relative to the weight of chitosan (NGF: bFGF: compound Hongqi injection = 1:1:1.5 weight ratio) into the above diluted chitosan solution and stir evenly to obtain chitosan-nerve growth factor mixed liquid. Soak the thin non-woven needle-punched calcium alginate fiber net (30 g / m2 specification, self-made) with a mixture of 2% acetic acid aqueous solution: acetone = 50:50, wash three times, dehydrate with pure acetone, air-dry, and then use carapace Fully impregnate the amine-nerve growth factor mixture for 20 minutes, take out the polytetrafluoroethylene cylindrical rod with a diameter of 6mm as the roller, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com