Benzofuran category compound, its preparing method and usage
A technology of benzopyran and compounds, which is applied in the synthesis of benzopyran compounds and the preparation of anti-type II diabetes drugs, which can solve the problems of high liver toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
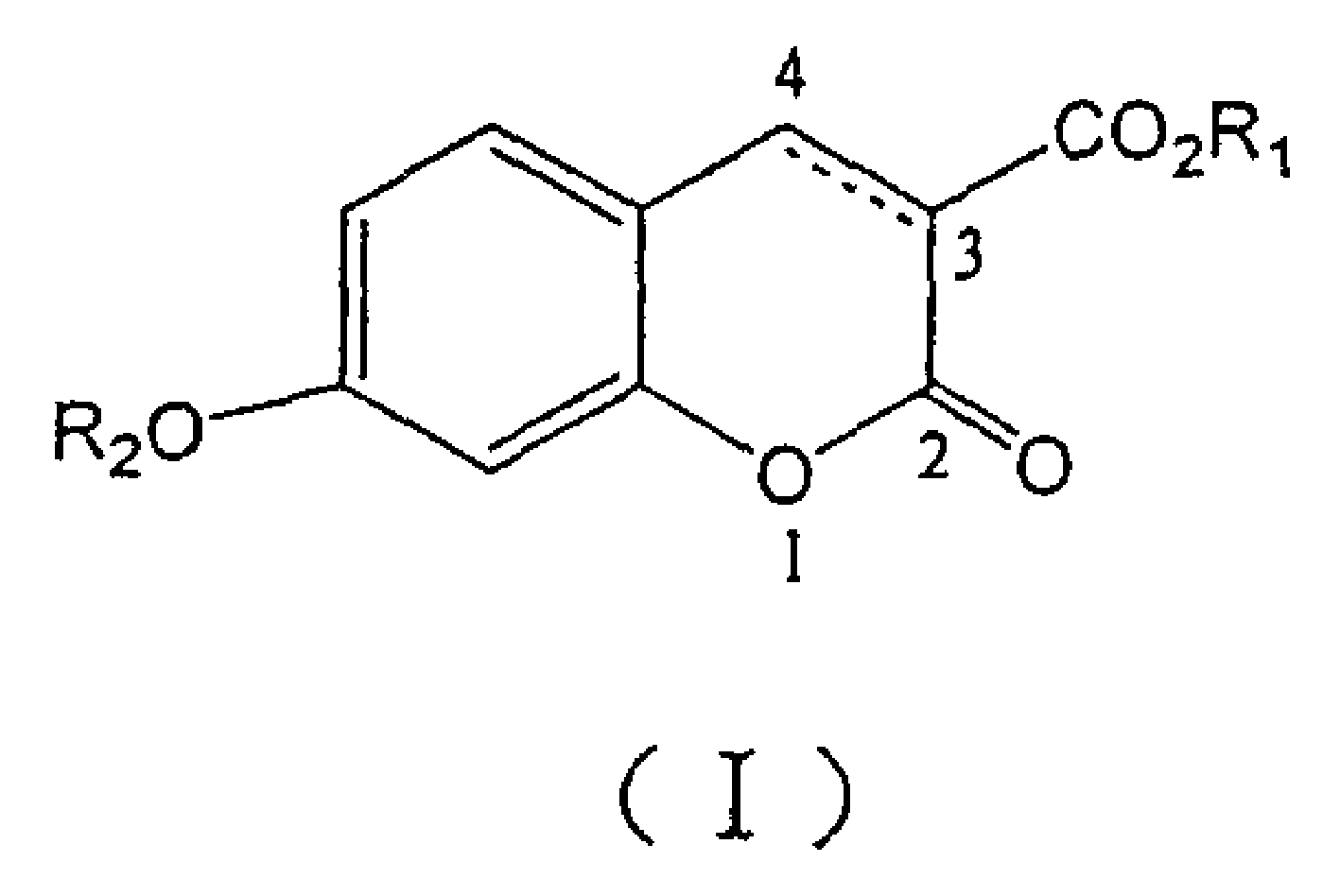
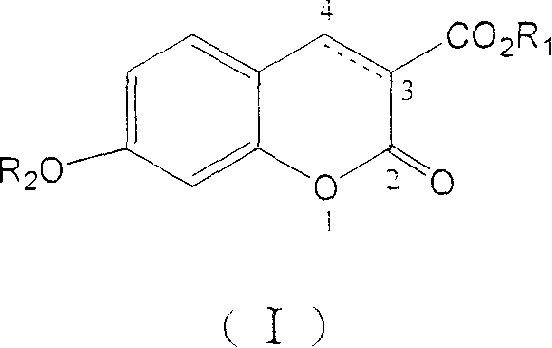
[0143] Example 1: 7-Benzyloxy-2-oxo-2H-1-benzopyran-3-carboxylic acid methyl ester (compound 1)
[0144] 7-Hydroxy-3-coumarin carboxylate methyl ester (0.2g, 1mmol) was dissolved in N,N'-dimethylformamide (2mL), added benzyl bromide (0.36ml, 3mmol) and finely ground carbonic acid Potassium (0.5g), stirred at 70°C for 12 hours. Add water (10 mL), extract with ethyl acetate (2×10 mL), combine ethyl acetate, wash with water, and dry over anhydrous sodium sulfate. Concentrate under reduced pressure to about 5 mL. A solid precipitated after standing, and was filtered with suction to obtain 0.21 g of the title compound, with a yield of 67.7%. m.p.129-130°C. 1 H NMR (CDCl 3 ): δ=3.92(s, 3H), 5.19(s, 2H), 6.85(d, J=2.4Hz, 1H), 6.95(dd, J=8.8Hz, 2.4Hz, 1H), 7.40(m, 5H ), 7.52(d, J=8.8Hz, 1H), 8.55(s, 1H); elemental analysis, C 18 h 14 o 5 (310): calculated value C, 69.68; H, 4.52. found value C, 69.63; H, 4.51; , 1026, 794.5, 725.1, 692.3, 636.4cm -1 ; EI-MS (m / z): 310 (12, M...
Embodiment 2
[0145] Example 2: 7-Benzyloxy-2-oxo-2H-1-chromene-3-carboxylic acid (Compound 2)
[0146] Compound 1 (0.1 g, 0.32 mmol) was dissolved in ethanol (0.5 mL), 10% aqueous sodium hydroxide solution (0.5 mL) was added, and the reaction was refluxed for 1 hour. After cooling, add concentrated hydrochloric acid (0.3 mL), continue stirring for 10 minutes, add water (5 mL), suction filter the produced solid, wash the filter cake with water, and dry to obtain the title compound 0.09 g, yield 90%. m.p.196-197°C. 1 H NMR (DMSO-d6): δ=5.25(s, 2H), 7.08(dd, J=2.2Hz, 8.8Hz, 1H), 7.12(d, J=2.2Hz, 1H), 7.30-7.50(m, 5H), 7.83(d, J=8.8Hz, 1H), 8.73(s, 1H); elemental analysis, C 17 h 12 O5(296): Calculated C, 68.92; H, 4.05; Found C, 68.50; H, 4.12; 732.8cm -1 ; EI-MS (m / z): 296 (4, M + ), 91(100).
Embodiment 3
[0147] Example 3: Methyl 7-(2-phenyl)ethoxy-2-oxo-2H-1-chromene-3-carboxylate (Compound 3)
[0148] Dissolve methyl 7-hydroxy-3-coumarincarboxylate (0.6g, 3mmol) and 2-phenylethanol (0.36mL, 3mmol) in anhydrous tetrahydrofuran (60mL), add triphenylphosphine (1.2g , 4.5mmol), diethyl azodicarboxylate (0.72mL, 4.5mmol) was slowly added dropwise at 0°C, and stirred at room temperature for 24 hours. The solvent was distilled off under reduced pressure, and the residue was precipitated in methanol as a white solid, which was recrystallized from methanol to obtain 0.45 g of the title compound, with a yield of 46.3%. m.p.100-101°C. 1 H NMR (CDCl 3 ): δ=3.12(t, J=6.8Hz, 2H), 3.92(s, 3H), 4.14(t, J=7.1Hz, 2H), 6.79(d, J=2.4Hz, 1H), 6.88(dd , J=2.4Hz, 8.8Hz, 1H), 7.21-7.35(m, 5H), 7.47(d, J=8.7Hz, 1H), 8.50(s, 1H); elemental analysis, C 19 h 16 o 5 (324): calculated value C, 70.37; H, 4.94. found value C, 70.25; H, 4.93; , 1114.7, 1018.2, 835, 749.5, 738.6, 700, 594cm -1 ; EI-MS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com