Pharmaceutical use of isolarisiresinol
A technology of isolarix and resinol, applied in the field of medicinal use of isolarix resinol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0019] Experimental Example 1: The present invention confirms that Radix Radix isatidis has the effective part of anti-endotoxemia effect through animal experiments
[0020] Test drug solution: the extracts obtained by the above extraction processes are diluted with water to prepare a drug solution containing 2 grams of crude drug per milliliter.
[0021] Test endotoxin: use the O 111 B 4 Escherichia coli, the endotoxin was prepared and purified by our laboratory, and the O 111 B 4 Escherichia coli endotoxin was used for titer calibration. The dosage range for the preparation of endotoxemia model mice is 15-18 mg per kg body weight.
[0022] Experimental animals: purchased from the Animal Breeding Farm of the Chinese Academy of Medical Sciences, Kunming species, secondary mice. Weight range: 18-22 grams, half male and half male. Randomly divided into groups according to body weight, with no less than 30 animals in each group. The t-test was carried out for the weight of...
experiment example 2
[0030] Experimental Example 2: Separation of active ingredient monomer compounds in the effective parts of the present invention: the effective extraction parts proved by animal experiments in the above table 1 have 3 main absorption peaks in high performance liquid chromatography, and 3 main absorption peaks in thin layer chromatography. A major spot is closely related to the curative effect. Since the content of the three main components in the ethyl acetate extraction part is relatively high, the ethyl acetate extraction part is subjected to column chromatography separation.
[0031] Materials: Silica gel for column chromatography, G plate and GF254 plate for thin layer chromatography produced by Qingdao Ocean Chemical Factory. Analytical reagents such as chloroform, methanol, petroleum ether, ethyl acetate, etc. from Beijing Yili Chemical Company. Development system: petroleum ether-chloroform-methanol=4:4:1, 2:4:1, chloroform-methanol=80:1, 40:1, 20:1.
[0032] Spectrum...
experiment example 3
[0034] Experimental example 3: the action intensity and ratio of each main component in the effective part of the present invention
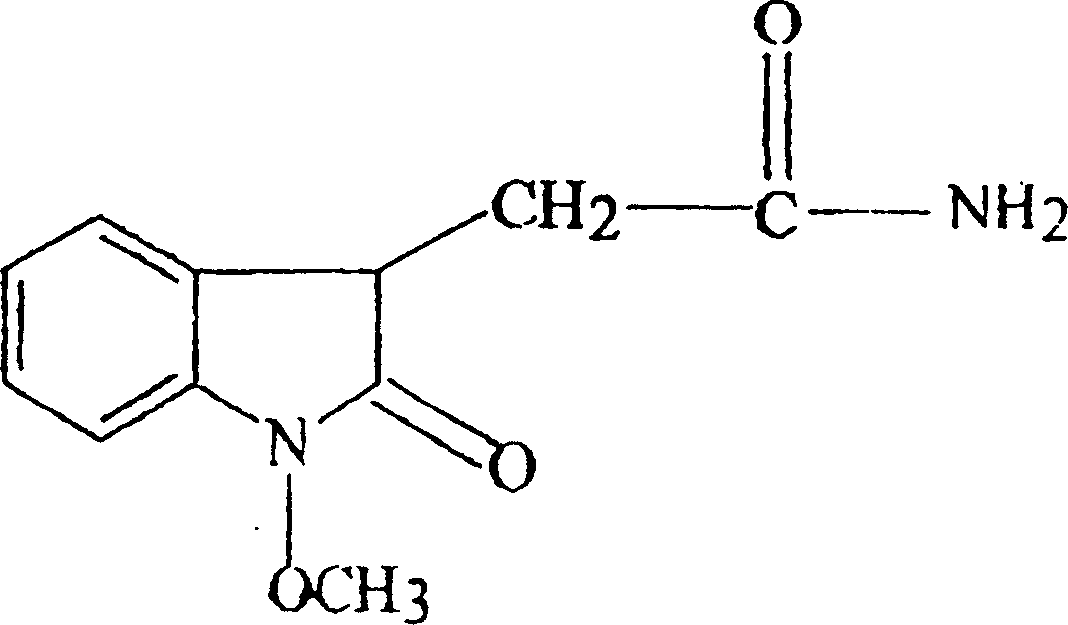
[0035] Preparation of the test solution: the ethyl acetate extract is separated by silica gel column chromatography, the solvent is recovered from the eluted part of chloroform and the eluted part of chloroform: methanol = 10: 1, and water is added to form a solution with a concentration of 2 g / ml of crude drug. Background solution, 5-hydroxymethylfurfural, isolaricinol ester, and 1-N-methoxy-2-oxo-indole-3-acetamide obtained by elution with different ratios of chloroform-methanol were added in different weight ratios In the background solution, select the best ratio. In order to ensure the stability of the active ingredients, the background solution of No. 3 effective part was adjusted to pH=2.5 with 5% hydrochloric acid, and the background solution of No. 6 effective part was adjusted to pH=4.5 with 5% sodium hydroxide. Experimental animals, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com