Loal color-lightening compound and its application method
一种组合物、局部的技术,应用在颜色调浅领域
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
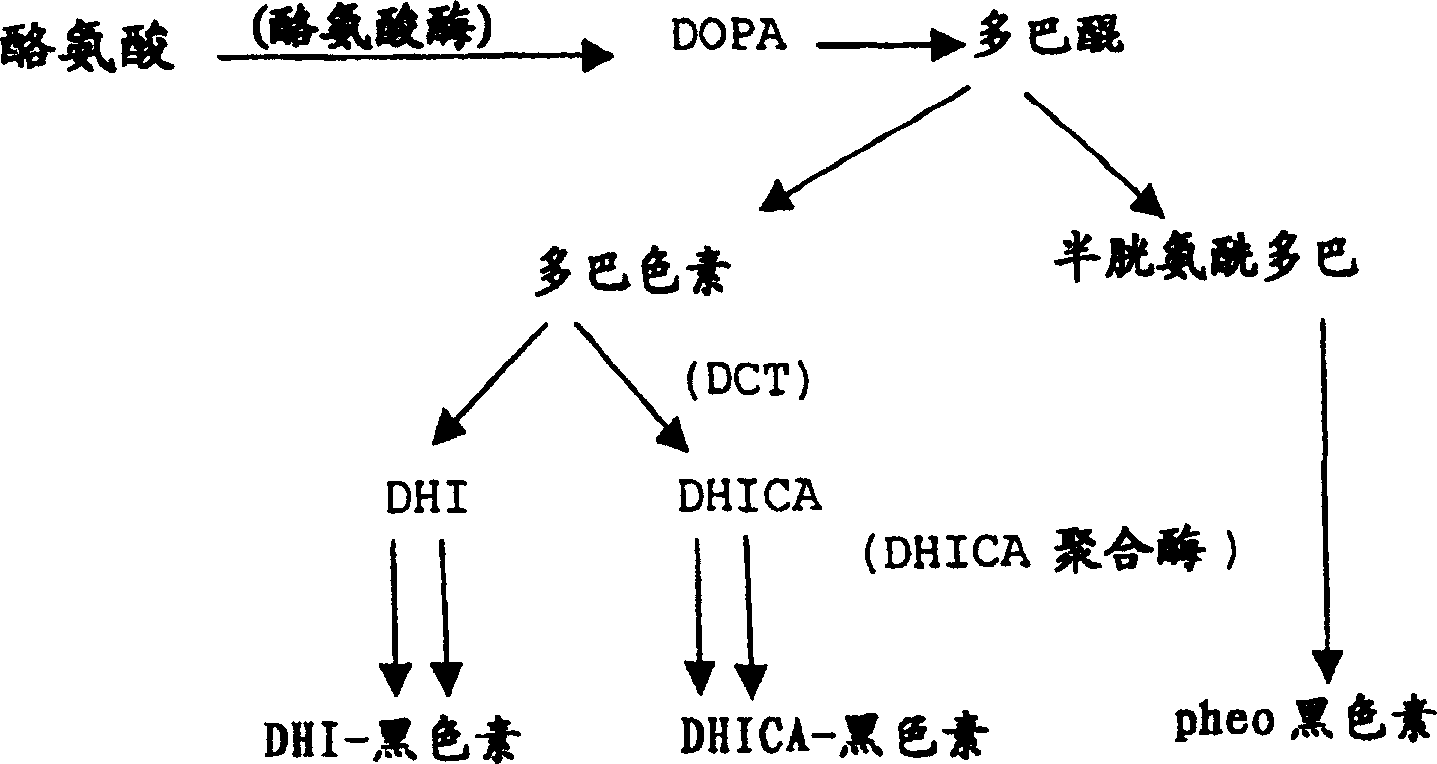
[0034] Embodiment 1: dopachrome tautomerase test (coconut juice)
[0035] Dopachrome tautomerase (DCT) activity was determined according to the method disclosed by Chakraborty et al., 1998, Effect of arbutin on melanogenic proteins in human melanocytes, Pigment Cell Res. 11:206-212. Specifically, frozen DOPA (0.5 mg per mL of 0.1M sodium phosphate buffer, pH 6.8) was mixed with Ag 2 O (30 mg Ag 2 (0: 1 mg DOPA) was mixed for about 1 minute and filtered through a 0.22 μm millipore filter. DCT was measured spectrophotometrically by adding a total of 0.1 mL of cell extract to 0.5 mL of freshly prepared dopachrome (-0.5 mg / mL). Reactions were carried out in plastic cuvettes at room temperature, after which the disappearance of absorption at 475 nm was followed. Phenylthiourea (1 mM) was added to the reaction mixture since the presence of tyrosinase in the cell extract may interfere with the assay. The percent conversion of dopachrome per mg of protein extract was calculated an...
Embodiment 2
[0036] Example 2: DHICA polymerase assay (coconut water)
[0037] DRICA polymerization was carried out according to the method in "Polymerization of 5,6-dihydroxyindole-2-Carboxylic acid to melanin by the pmel17 / silver locus protein" published by Chakraborty et al. in Eur.J.Biochem.236:180-188 in 1996 Enzyme assay. Specifically, cell extracts (0.5 mL, 150-200 μg protein) were passed through a wheat germ agglutinin column (1 mL bed volume) equilibrated with lysis buffer. The bound material, which contains crude DHICA aggregation factor and other melanogenic proteins, was eluted with 0.5 mL of 1M N-acetylglucosamine. In a 0.5 ml reaction mixture containing the enzyme preparation to be assayed (20 μg protein from wheat germ agglutinin eluate) or the appropriate buffer blank, DRICA (0.5 mM), and 100 mM sodium phosphate buffer, pH 7.0 . It also includes phenylthiourea, which is used to inhibit endogenous tyrosinase activity in the formulation.
[0038] At time points T=0 and T=...
Embodiment 3
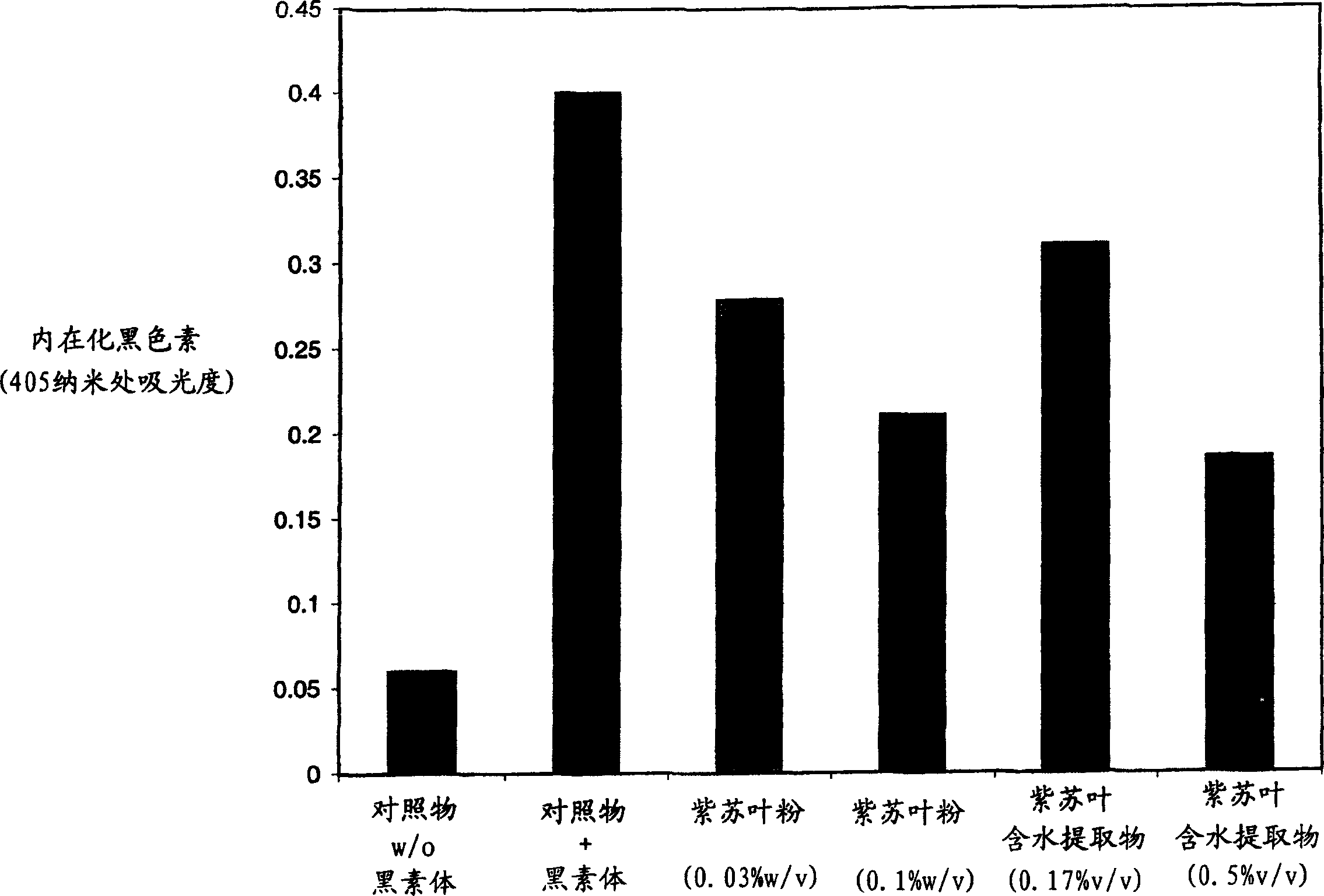
[0039] Example 3: Melanosome Uptake Assay (Perilla Leaf Extract)
[0040] Melanosome isolation
[0041] Congenital cultures of B16 melanocytes produce moderate amounts of melanosomes. However, to induce increased melanosome formation in this cell line, hemiconnate (60%) cultured B16 cells were treated for approximately 36 hours with normal growth medium supplemented with 10 mM ammonium chloride (final concentration). The medium was then aspirated and the hyperpigmented cells were washed (2 x 2 ml) with distilled water to provide hypotonic stress to the cells. Aliquots (2 mL) of hypotonic lysis solution (0.02% NP-40 in water) were added to each plate and the plates were incubated at room temperature for approximately 5 minutes. Cell lysis was followed by light microscopy, and cytoplasm from three (3) dishes was submerged in a 15 ml conical tube and centrifuged at approximately (200xg) for 5 minutes to remove cellular debris . The resulting melanosome-containing supernatant ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com