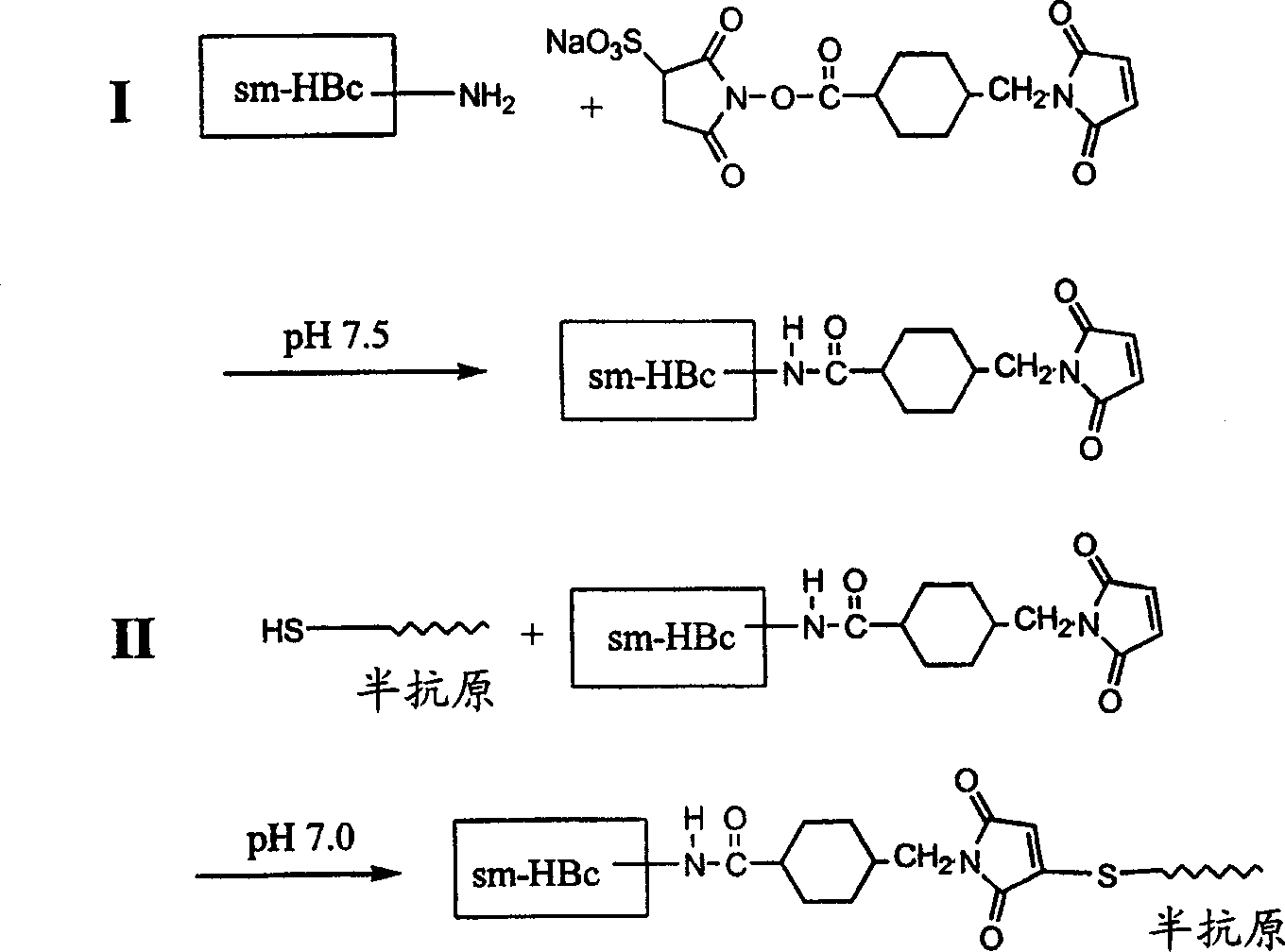
Strategically modified hepatitis b core proteins and their derivatives
A core protein and strategic technology, applied in anti-animal/human immunoglobulins, microorganisms, immunoglobulins, etc., can solve the problem that amino acid side chains do not show chemical reactivity, and achieve the effect of enhancing chemical reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0092] Ⅲ. Preparation of strategically modified core protein
[0093] The strategic modification of hepatitis B core protein is usually performed at the DNA level by methods known in the art, for example, by inserting codons corresponding to the amino acid sequence to be inserted. The manipulated gene is then expressed in a convenient system known in the art, for example in a viral culture of infected immortalized cells.
[0094] The method of preparing HBcAg protein in general and the method of preparing pre-core, core and HBeAg protein in particular are well known in the art. The same method can easily be modified into a method for separating the modified core protein particles of the present invention. In addition, HBcAg and HBeAg can be produced by a variety of well-known recombinant DNA techniques. See, for example, U.S. Patent No. 4,356,270 to Itakura and 4,563,423 to Murray et al. These recombinant DNA technologies can easily be adapted into methods for producing the modifi...
Embodiment 1
[0161] Example 1: Construction of modified hepatitis B core protein expression vector.
[0162] Site-directed mutagenesis introduced a lysine codon (TTT) between amino acids D78 and P79 of the HBc gene, and also introduced EcoRI (GAATTC) and SacⅠ (GAGCTC) restriction endonuclease sites to facilitate genetic insertion of other codons To produce strategically modified hybrid HBc particles. The amino acid residue sequence of such an inserted sequence is GIQKEL, where GIQ is the product of EcoRI site and EL is the product of SacI site. Therefore, the length of the strategically modified hepatitis B core protein is 155 amino acid residues. The construction of pKK322-HBc155-K81 expression plasmid is described below.
[0163] The 5'end (bases 1-234, amino acids 1-78) of the HBc gene was amplified with oligonucleotide primers P1F (SEQ ID NO: 17) and P1R (SEQ ID NO: 18, on the complementary strand) and At the same time, a NcoI restriction site (CCATGG) was introduced at the 5'end of the am...
Embodiment 2
[0165] Example 2: Purification of modified hepatitis B core protein particles
[0166] E. coli, usually E. coli BLR or BL21 from Novagen (Madison, Wisconsin) or E. coli TB11 from Amersham (Arlington Heights, Illinois), expresses the strategically modified HBc protein of Example 1. HBc155-K81 transfected with E. coli is the expression plasmid pKK332-HBc155-K81. The strategically modified HBc particles were purified by an established method using Sepharose CL-4B chromatography. Since the particles are purified in a predictable manner, simple spectrophotometry (OD 280 ) Plus the use of SDS-PAGE to detect the purity of each fraction before collection to monitor the elution of particles is sufficient to allow conventional purification to obtain high-yield electrophoretic pure particles (5-120mg / L cell culture). The spherical structure of the purified strategically modified hepatitis B core particles can be clearly seen under the electron microscope.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com