Ethinyl estradiol gas permeable absorbing paste
An ethinyl estradiol and transdermal technology is applied in the field of pharmacy to achieve the effects of prolonging the action time of the drug, meeting the treatment requirements and increasing the transdermal rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
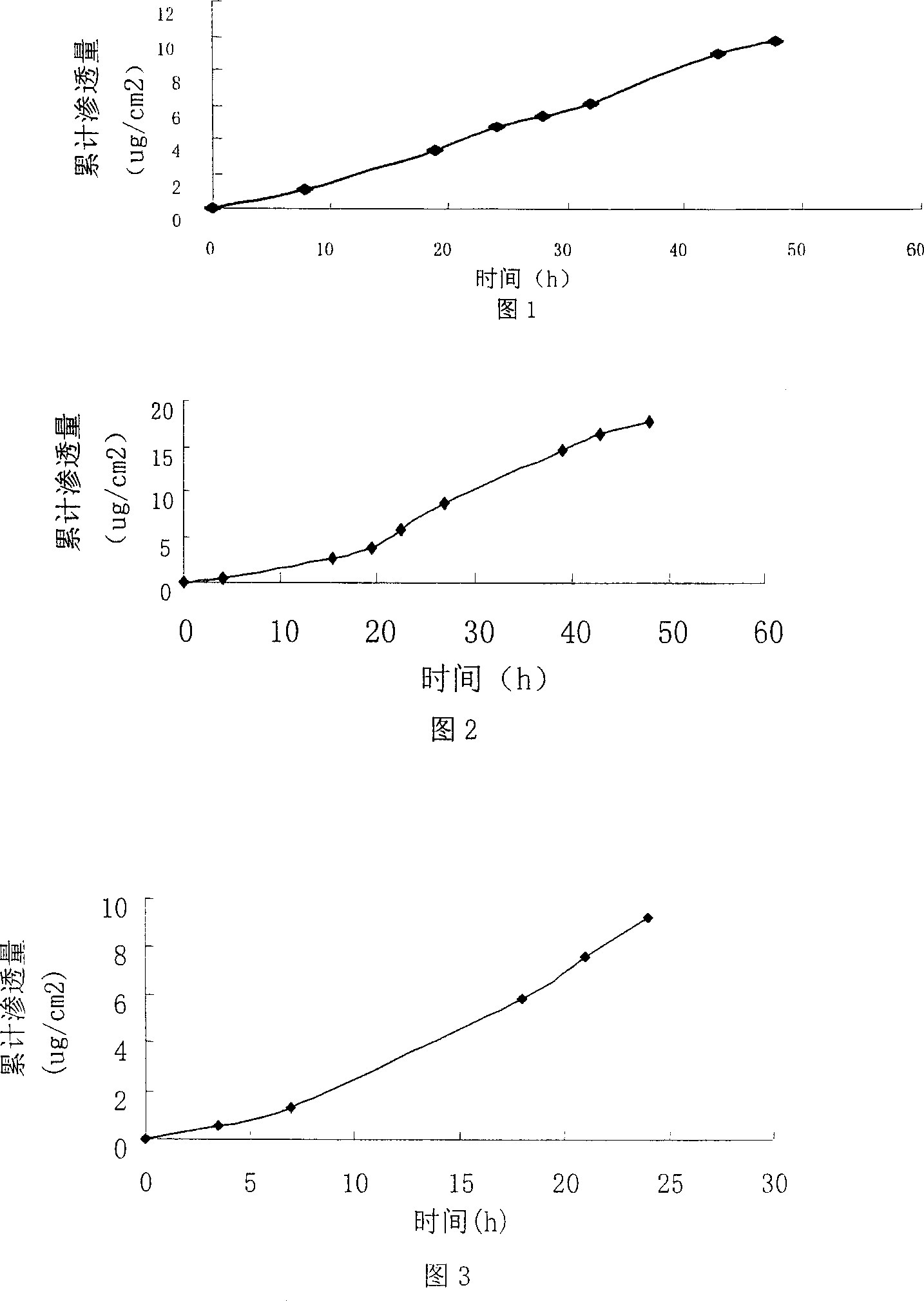
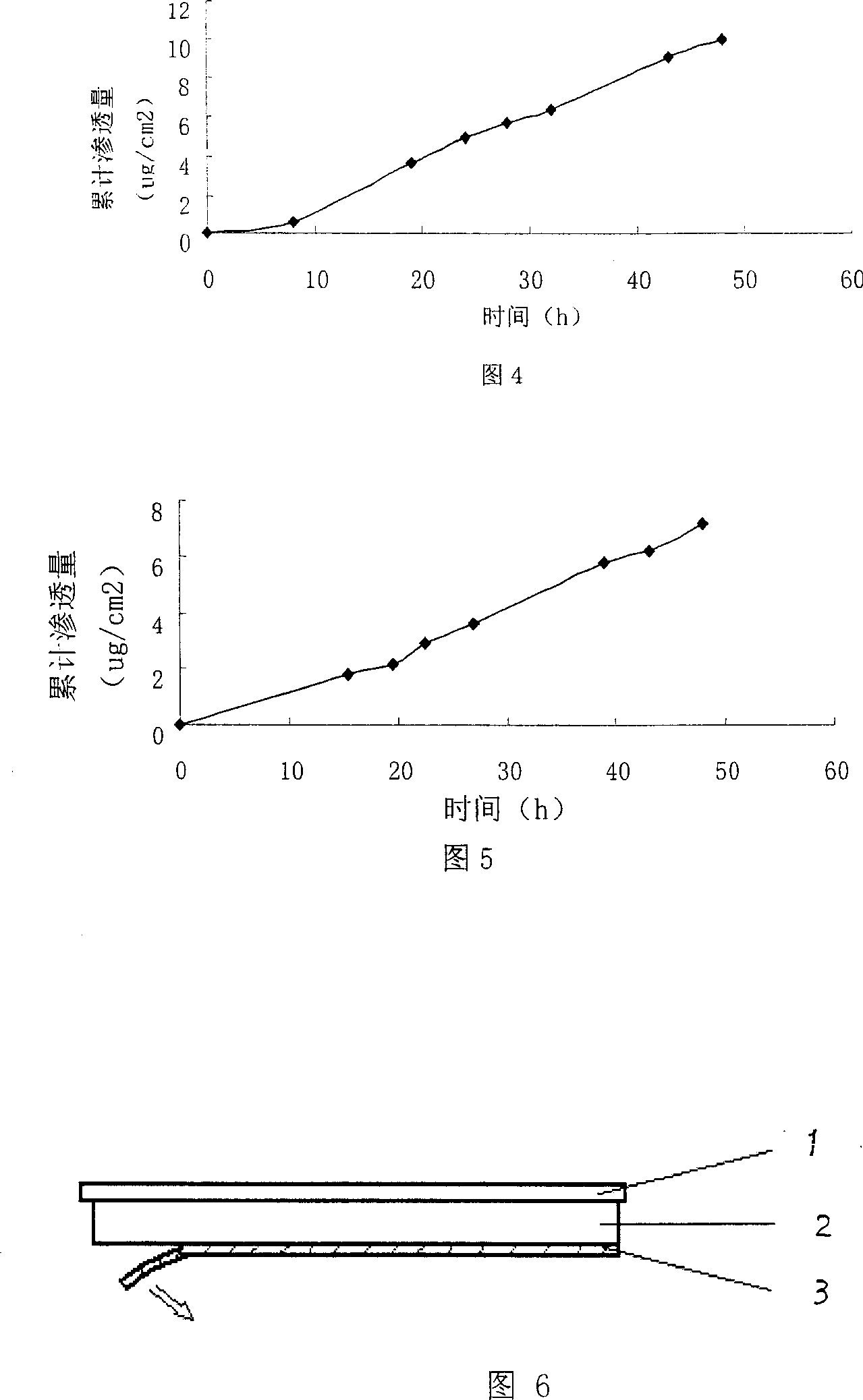
[0020] Polyacrylate pressure sensitive adhesive (PSA) 390mg, dibutyl sebacate 175mg, succinic acid 35mg, drug ethinyl estradiol 20mg, oleic acid 160mg, solvent (acetone:isopropanol:ethanol=21:2.3:11.7) 2400mg , together into the bottle, the bottle mouth is closed, until completely dissolved, spread on 40cm 2 on the backing layer, followed by drying at 60°C for 30min. The dried matrix layer contains 50% polyacrylate, 2.6% ethinyl estradiol, and 20.5% oleic acid. After cooling, it covers the upper lining layer. The improved Franz diffusion cell device is used to measure its permeability to human skin in vitro. The receiving solution is 20% polyethylene glycol 400 (PEG400) saline solution in water. Use HPLC to measure ethinyl estradiol, the wavelength is 281nm, the mobile phase is 70% methanol, 30% water, the flow rate is 1ml / min, for ethinyl estradiol, the retention time is about 5.5min, and the permeation rate is 0.76ug / cm 2 h. Fig. 1 is the cumulative drug penetration-time ...
Embodiment 2
[0022] Polyacrylate pressure sensitive adhesive (PSA) 390mg, dibutyl sebacate 175mg, succinic acid 35mg, drug ethinyl estradiol 20mg, oleic acid 100mg, solvent (acetone:isopropanol:ethanol=21:2.3:11.7) 2400mg , as in 1 operation, the matrix layer after drying contains 54% polyacrylate, 2.8% ethinyl estradiol, 13.9% oleic acid, covers the upper lining layer after cooling, and uses the modified Franz diffusion cell device to measure its penetration to human skin in vitro rate, the receiving fluid is 20% polyethylene glycol 400 (PEG400) saline aqueous solution, and the penetration rate is 0.50ug / cm 2 h. Fig. 2 is the cumulative drug penetration-time curve of this embodiment.
Embodiment 3
[0024] Polyacrylate pressure sensitive adhesive (PSA) 300mg, dibutyl sebacate 135mg, succinic acid 27mg, drug ethinyl estradiol 20mg, oleic acid 230mg, solvent (acetone:isopropanol:ethanol=21:2.3:11.7) 1848mg , as in 1 operation, the matrix layer after drying contains polyacrylate 42%, ethinyl estradiol 2.8%, oleic acid 32.3%, after cooling, cover the upper lining layer, and adopt the modified Franz diffusion cell device to measure its penetration to human skin in vitro rate, the receiving fluid is 20% polyethylene glycol 400 (PEG400) saline aqueous solution, and the penetration rate is 0.43ug / cm 2 h. Fig. 3 is the cumulative drug penetration-time curve of this embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com