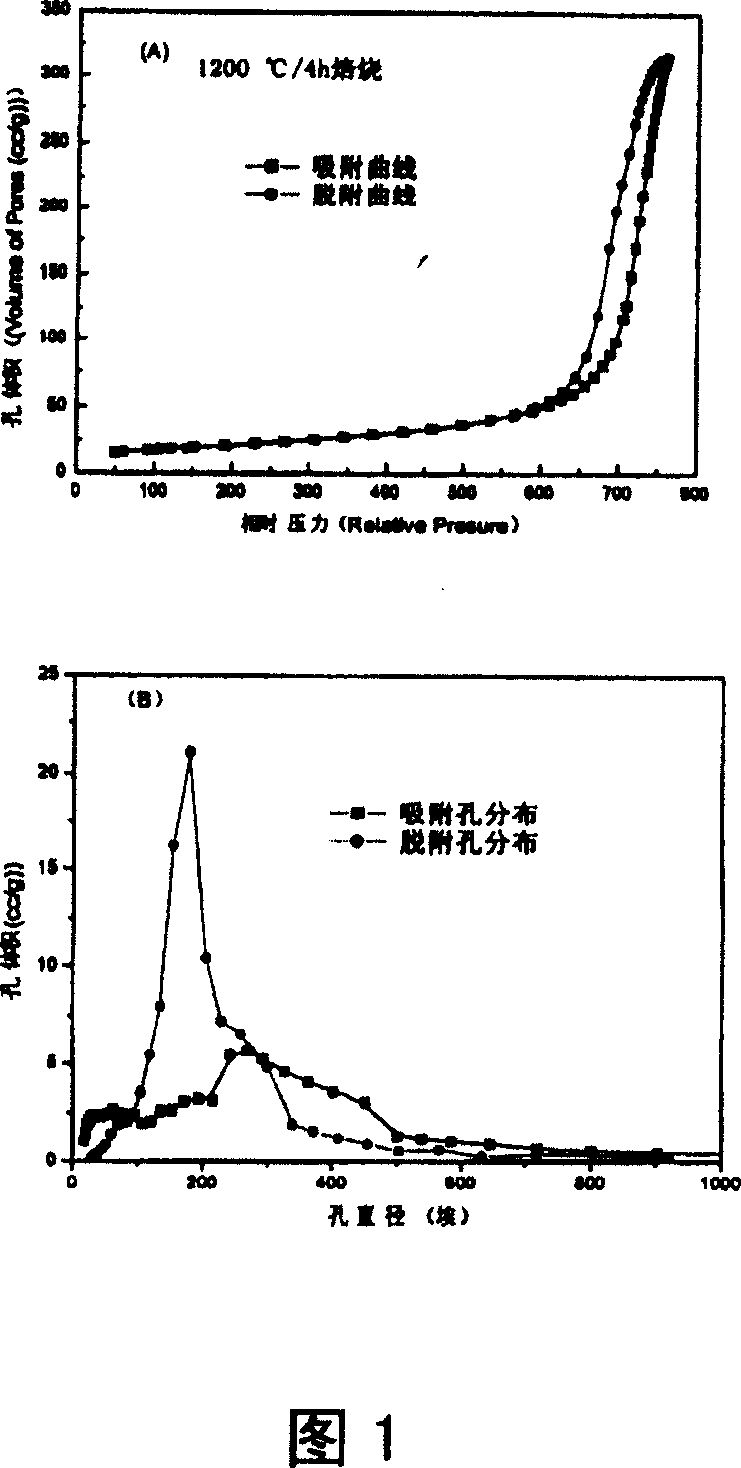
Preparation of transition metal substituted hexaaluminate as catalyst for natural gas combustion
A technology of combustion catalyst and hexaaluminate, which is applied in the direction of catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc. Solve problems such as difficulty in salt preparation, and achieve the effect of low cost and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0038] Example 1. Using the above co-precipitation method to prepare Ba 1-x La x Fe y Al 12-y O 19-δ :
[0039] Configuration series stoichiometric Fe(NO 3 ) 3 And Al(NO 3 ) 3 , La(NO 3 ) 3 , Ba(NO 3 ) 2 Mix the solution, dilute nitric acid to adjust the pH = 1 or so, and configure the saturated (NH 4 ) 2 CO 3 Solution. Pour the mixed salt solution quickly into saturated (NH 4 ) 2 CO 3 The solution is stirred vigorously, the temperature of the system is controlled at 60°C, and the pH value is controlled at 7.5-8.0. The resulting brown slurry is continuously stirred vigorously for 2 hours and aged at 60°C for 3 hours; washed to remove NO 3 - The resulting filter cake is dried at 60°C and dried at 120°C. The above samples were fired at 500°C, 800°C, 1100°C, and 1200°C for 3h. Label the resulting catalysts as BF i A n -t, where A, B, F, I, t respectively represent aluminum (Al), barium (Ba), iron (Fe) atoms, i represents the number of iron (Fe) atoms, and n represents the number of ...
example 2
[0042] Example 2. Preparation of Ba by the above co-precipitation-hydrothermal method 1-x La x Fe y Al 12-y O 19-δ :
[0043] Configuration series stoichiometric Fe(NO 3 ) 3 And Al(NO 3 ) 3 , Ba(NO 3 ) 2 Mix the solution, dilute nitric acid to adjust the pH = 1 or so, and configure the saturated (NH 4 ) 2 CO 3 Solution or saturated urea solution. Pour the mixed salt solution quickly into saturated (NH 4 ) 2 CO 3 Solution or excess saturated urea solution, stir vigorously, the temperature of the system is controlled at 60℃, the pH value is controlled at 7.5-8.0, the brown slurry obtained is aged at 60℃ for 3h, and then transferred to the reactor for treatment at 120℃ for 3h; the resulting precipitate is washed Remove NO 3 - The resulting filter cake was dried at 120°C. The above samples were fired at 500°C, 800°C, 1100°C, and 1200°C for 3h. Label the resulting catalysts as BF i A n -t, where A, B, F, I, t respectively represent aluminum (Al), barium (Ba), iron (Fe) atoms, i repres...
example 3
[0044] Example 3. Preparation of Ba using the above hydrothermal method 1-x La x Fe y Al 12-y O 19-δ :
[0045] Configuration series stoichiometric Fe(NO 3 ) 3 And Al(NO 3 ) 3 , Ba(NO 3 ) 2 Mix the solution, dilute nitric acid to adjust the pH = 1 or so, and configure the saturated (NH 4 ) 2 CO 3 Solution or saturated urea solution. Pour the mixed salt solution quickly into saturated (NH 4 ) 2 CO 3 Solution or excess saturated urea solution, stir vigorously, the temperature of the system is controlled at 60℃, the pH value is controlled at 7.5-8.0, the resulting brown slurry is transferred to the reactor at 120℃ for 3h; the resulting precipitate is washed to remove NO 3 - The resulting filter cake is dried at 60°C and dried at 120°C. The above samples were fired at 500°C, 800°C, 1100°C, and 1200°C for 3h. Label the resulting catalysts as BF i A n -t, where A, B, F, I, t respectively represent aluminum (Al), barium (Ba), iron (Fe) atoms, i represents the number of iron (Fe) atoms, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com